Label: TECHNELITE- technetium tc99m generator injection, solution
- NDC Code(s): 11994-090-01, 11994-090-03, 11994-090-04, 11994-090-05, view more
- Packager: Lantheus Medical Imaging, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR DIAGNOSTIC USE - For the Production of Sodium Pertechnetate Tc 99m Injection
-
DESCRIPTIONDESCRIPTION: Sodium Pertechnetate Tc 99m Injection, as eluted according to the elution instructions with Lantheus Medical Imaging, Inc. TECHNELITE®, Technetium Tc 99m Generator, is in 0.9 ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: The pertechnetate ion distributes in the body similarly to the iodide ion but is not organified when trapped in the thyroid gland. It also concentrates in the choroid ...
-
INDICATIONS AND USAGE:The Technelite generator is a source of sodium pertechnetate Tc 99m for use in the preparation of FDA-approved diagnostic radiopharmaceuticals, as described in the labeling of these diagnostic ...
-
CONTRAINDICATIONSCONTRAINDICATIONS: None known.
-
WARNINGSWARNINGS: Radiation risks associated with the use of Sodium Pertechnetate Tc 99m Injection are greater in children than in adults and, in general, the younger the child, the greater the risk ...
-
PRECAUTIONS:General - As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient consistent with proper patient management and to ensure minimum radiation ...
-
ADVERSE REACTIONSADVERSE REACTIONS: Allergic reactions including anaphylaxis have been reported infrequently following the administration of Sodium Pertechnetate Tc 99m Injection.
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Sodium Pertechnetate Tc 99m Injection is usually administered by intravascular injection. For imaging the urinary bladder and ureters (direct isotopic cystography) ...
-
HOW SUPPLIEDHOW SUPPLIED: Lantheus Medical Imaging TechneLite®, (Technetium Tc 99m Generator) for the Production of Sodium Pertechnetate Tc 99m Injection is supplied in a multi-dose container and is ...
-
STORAGE AND HANDLINGSTORAGE: Store generator and Sodium Pertechnetate Tc 99m Injection at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
-
SPL UNCLASSIFIED SECTIONEXPIRATION: The expiration time of the Sodium Pertechnetate Tc 99m solution is not later than 12 hours after elution. If the eluate is to be used to reconstitute a kit for the preparation of a ...
-
SPL UNCLASSIFIED SECTIONELUTION INSTRUCTIONS - TOTAL ELUTION METHOD - Waterproof gloves should be worn during elution. Remove dust (clear plastic) cover of generator. Perform all subsequent operations ...
-
SPL UNCLASSIFIED SECTIONDISPOSAL: All components shipped with the TECHNELITE®, Technetium Tc 99m Generator should be monitored for contamination prior to disposing into routine trash systems. The Technetium Tc 99m ...
-
SPL UNCLASSIFIED SECTIONLantheus - Medical Imaging - 331 Treble Cove Road - N. Billerica, MA 01862 USA - For Ordering Call Toll-Free: 800-299-3431 All other business: 800-362-2668 - (In ...
-
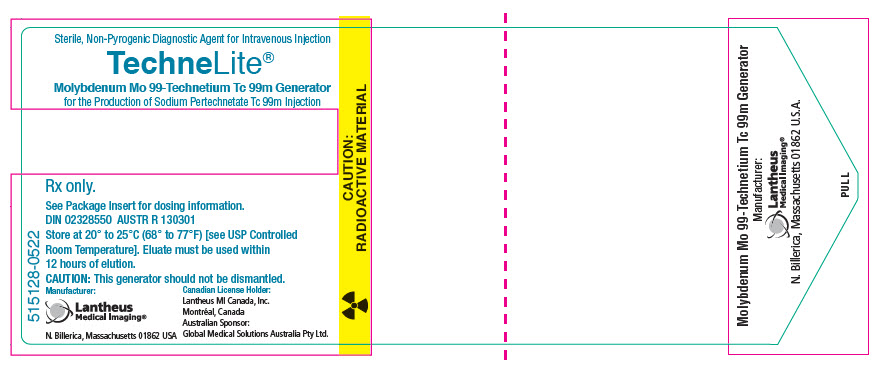
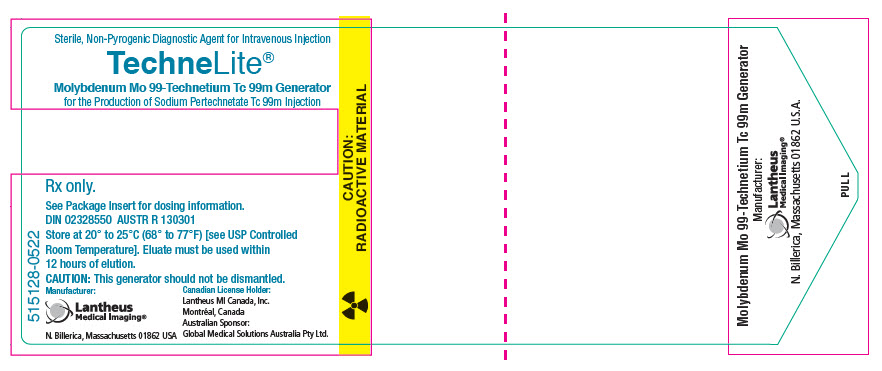
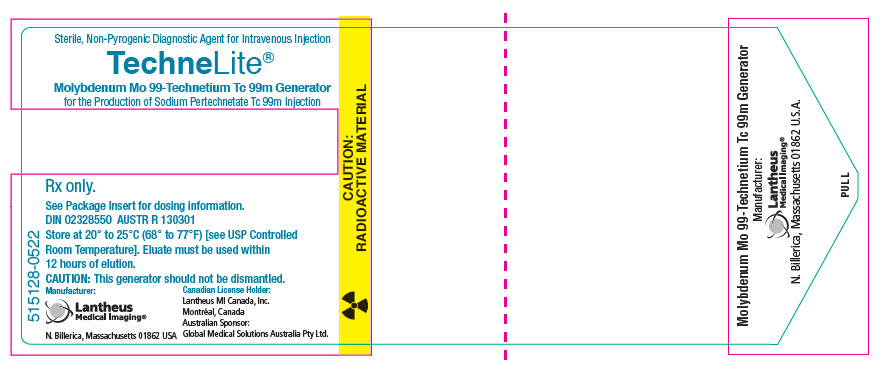
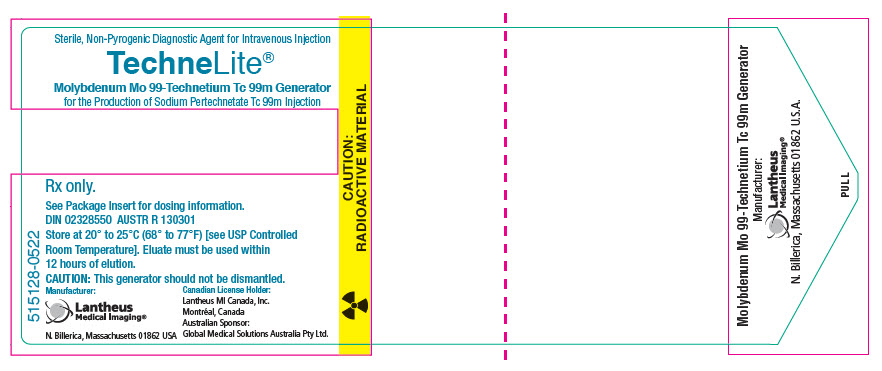
PRINCIPAL DISPLAY PANEL - Generator Label - NDC 11994-090Sterile, Non-Pyrogenic Diagnostic Agent for Intravenous Injection - TechneLite® Technetium Tc 99m Generator - for the Production of Sodium Pertechnetate Tc 99m Injection - Rx only. See Package Insert ...
-
PRINCIPAL DISPLAY PANEL - Generator Label - NDC 11994-091Sterile, Non-Pyrogenic Diagnostic Agent for Intravenous Injection - TechneLite® Technetium Tc 99m Generator - for the Production of Sodium Pertechnetate Tc 99m Injection - Rx only. See Package Insert ...
-
INGREDIENTS AND APPEARANCEProduct Information