Label: TAZVERIK- tazemetostat tablet, film coated
- NDC Code(s): 72607-100-00
- Packager: Epizyme, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAZVERIK® safely and effectively. See full prescribing information for TAZVERIK. TAZVERIK (tazemetostat) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Epithelioid Sarcoma - TAZVERIK is indicated for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients with R/R FL for treatment with TAZVERIK based on the presence of EZH2 mutation of codons Y646, A682, or A692 in tumor specimens [see Clinical Studies ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 200 mg film-coated, red, round, biconvex shape and debossed with "EZM 200" on one side and plain on the other.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Secondary Malignancies - The risk of developing secondary malignancies is increased following treatment with TAZVERIK. Across clinical trials of 758 adults who received TAZVERIK 800 mg twice ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Secondary Malignancies [see Warnings and Precautions (5.1)]. 6.1 Clinical Trial Experience - Because ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on TAZVERIK - Strong or Moderate CYP3A Inhibitors - Coadministration of TAZVERIK with a strong or moderate CYP3A inhibitor increases tazemetostat plasma ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], TAZVERIK can cause fetal harm when administered to pregnant ...
-
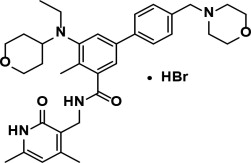
11 DESCRIPTIONTazemetostat is a methyltransferase inhibitor. Tazemetostat hydrobromide has the following chemical name: [1,1'-Biphenyl]-3-carboxamide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tazemetostat is an inhibitor of the methyltransferase, EZH2, and some EZH2 gain-of-function mutations including Y646X, A682G, and A692V. Tazemetostat also inhibited ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Dedicated carcinogenicity studies were not conducted with tazemetostat, but T-LBL, MDS, AML, and B-ALL have been reported clinically ...
-
14 CLINICAL STUDIES14.1 Epithelioid Sarcoma - The efficacy of TAZVERIK was evaluated in an open-label, single-arm cohort (Cohort 5) of a multi-center study (Study EZH-202, NCT02601950) in patients with ...
-
15 REFERENCESCheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTAZVERIK 200 mg film-coated tablets are red, round, biconvex shape and debossed with "EZM 200" on one side and plain on the other. TAZVERIK is available in: Bottles of 240 tablets with a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Secondary Malignancies - Advise patients of the increased risk of secondary hematologic malignancies. Advise ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: November/2023 - MEDICATION GUIDE - TAZVERIK® (taz vayr′ ik) (tazemetostat) tablets - What ...
-
Principal Display Panel - 200 mg Bottle LabelNDC 72607-100-00 - 240 Tablets - TAZVERIK® (tazemetostat) tablets - 200 mg - Rx only - Dispense the Medication Guide, attached or - provided separately, to each patient pursuant - to Federal Law. Epizyme
-
INGREDIENTS AND APPEARANCEProduct Information