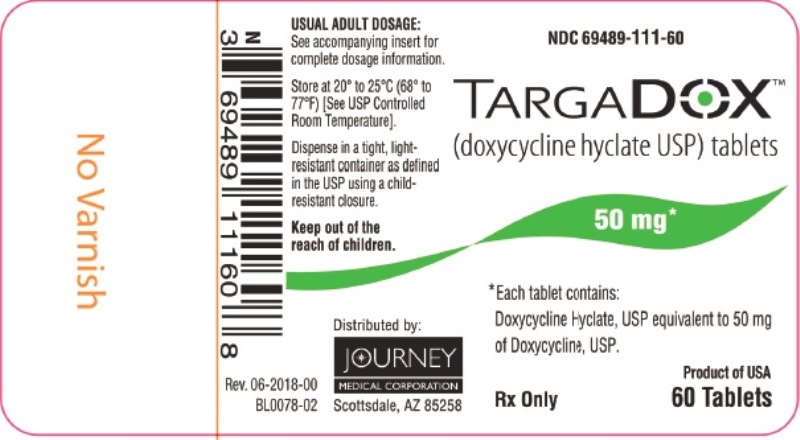
Label: TARGADOX- doxycycline hyclate tablet
- NDC Code(s): 69489-111-05, 69489-111-60
- Packager: Journey Medical Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of TARGADOX® and other antibacterial drugs, TARGADOX® should be used only to treat or prevent ...
-
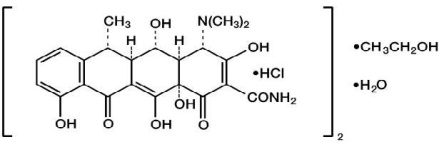
DESCRIPTIONDoxycycline is an antibacterial drug synthetically derived from oxytetracycline, and is available as doxycycline hyclate tablets, USP for oral administration. The chemical designation of ...
-
CLINICAL PHARMACOLOGYTetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations ...
-
INDICATIONS AND USAGE To reduce the development of drug-resistant bacteria and maintain effectiveness of TARGADOX® and other antibacterial drugs, TARGADOX® should be used only to treat or prevent infections that are ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGSThe use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth ...
-
PRECAUTIONSGeneral - As with other antibacterial drugs, use of doxycycline hyclate tablets may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, doxycycline ...
-
ADVERSE REACTIONSDue to oral doxycycline’s virtually complete absorption, side effects of the lower bowel, particularly diarrhea, have been infrequent. The following adverse reactions have been observed in ...
-
OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases ...
-
DOSAGE AND ADMINISTRATIONThe usual dosage and frequency of administration of doxycycline differs from that of the other tetracyclines. Exceeding the recommended dosage may result in an increased incidence of side ...
-
HOW SUPPLIEDTARGADOX® (doxycycline hyclate tablets, USP) equivalent to 50 mg of doxycycline: Light beige color, round convex, film coated tablets debossed “050” below, “J” on one side and plain on the ...
-
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGYHyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO4, and methacycline; in ...
-
REFERENCES1. Friedman JM and Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore, MD: The Johns Hopkins University Press, 2000: 149–195. 2. Cziezel AE and Rockenbauer M ...
-
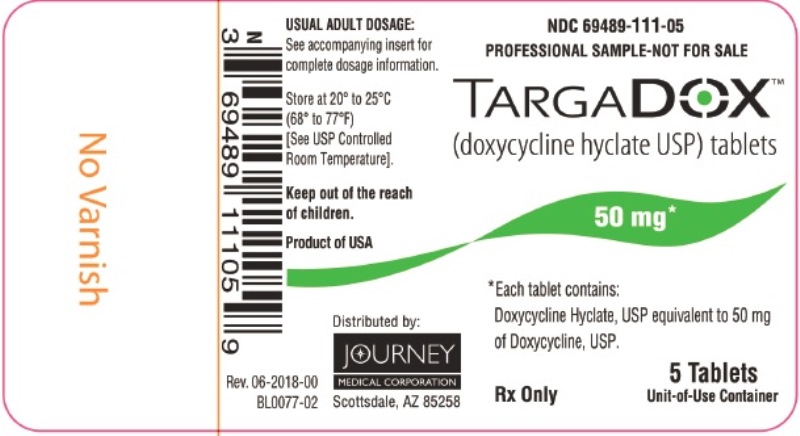
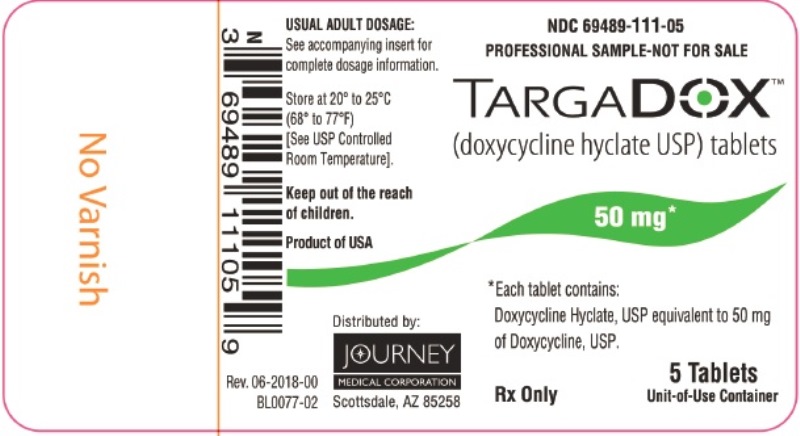
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL– TARGADOX 50 mg, 5 TabletsTARGADOX - (doxycycline hyclate USP) tablets - PROFESSIONAL SAMPLE-NOT FOR SALE - 50 mg 5ct - Rx Only
-
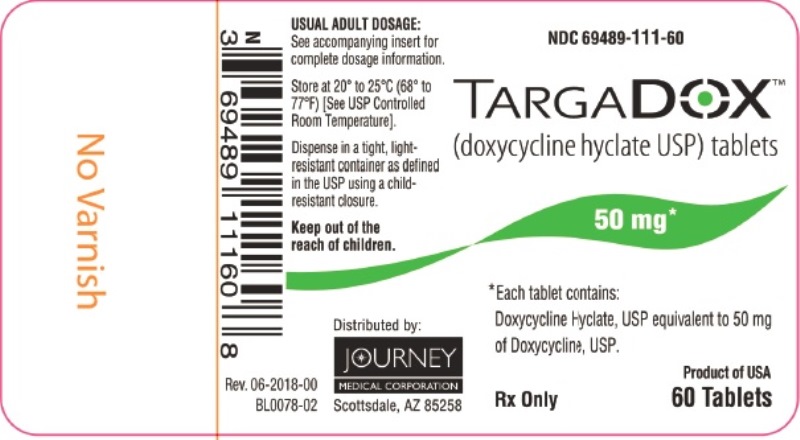
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – TARGADOX 50 mg, 60 TabletsTARGADOX - (doxycycline hyclate USP) tablets - 50 mg 60ct - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information