Label: TABRECTA- capmatinib tablet, film coated
- NDC Code(s): 0078-0709-56, 0078-0709-94, 0078-0716-56, 0078-0716-94
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TABRECTA safely and effectively. See full prescribing information for TABRECTA. TABRECTA® (capmatinib) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETABRECTA is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients for treatment with TABRECTA based on the presence of a mutation that leads to MET exon 14 skipping in tumor or plasma specimens [see Clinical Studies ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 150 mg: pale orange brown, ovaloid, curved film-coated with beveled edges, unscored, debossed with ‘DU’ on one side and ‘NVR’ on the other side - 200 mg: yellow, ovaloid, curved ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Interstitial Lung Disease (ILD)/Pneumonitis - ILD/pneumonitis, which can be fatal, occurred in patients treated with TABRECTA [see Adverse Reactions (6.1)]. ILD/pneumonitis occurred in ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: ILD/Pneumonitis [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on TABRECTA - Strong CYP3A Inhibitors - Coadministration of TABRECTA with a strong CYP3A inhibitor increased capmatinib exposure, which may increase the incidence ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], TABRECTA can cause fetal harm when administered to a ...
-
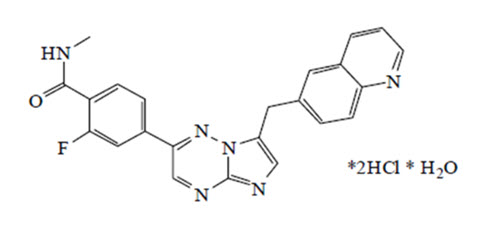
11 DESCRIPTIONCapmatinib is a kinase inhibitor. The chemical name is 2-Fluoro-N-methyl-4-[7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide—hydrogen chloride—water (1/2/1). The molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Capmatinib is a kinase inhibitor that targets MET, including the mutant variant produced by exon 14 skipping. MET exon 14 skipping results in a protein with a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies were not conducted with capmatinib. Capmatinib was not mutagenic in an in vitro bacterial reverse mutation ...
-
14 CLINICAL STUDIESMetastatic NSCLC with a Mutation that Leads to MET Exon 14 Skipping - The efficacy of TABRECTA was evaluated in GEOMETRY mono-1, a multicenter, non-randomized, open-label, multi-cohort study ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - TABRECTA (capmatinib) 150 mg and 200 mg tablets - StrengthDescriptionTablets per bottleNDC number - 150 mgPale orange brown, ovaloid, curved film-coated tablet with beveled ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Interstitial Lung Disease (ILD)/Pneumonitis - Inform patients of the risks of severe or fatal ILD/pneumonitis ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: March 2025 - PATIENT INFORMATION - TABRECTA® (ta brek tah) (capmatinib) tablets - What is ...
-
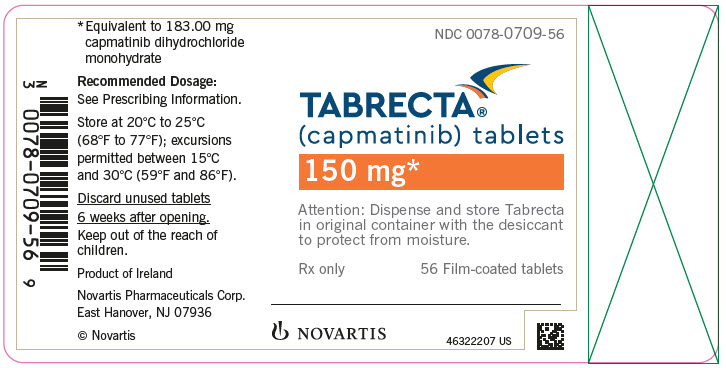
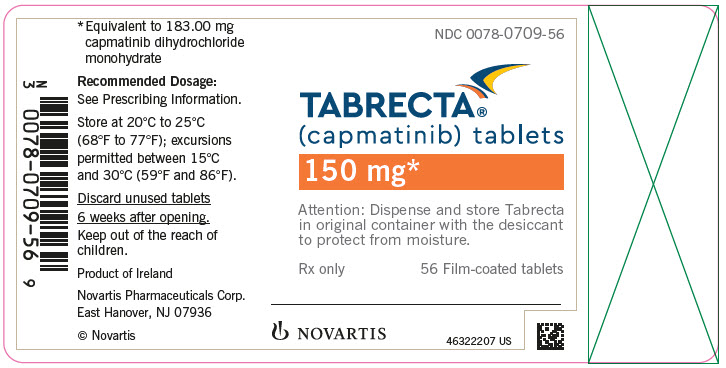
PRINCIPAL DISPLAY PANELNDC 0078-0709-56 - TABRECTA® (capmatinib) tablets - 150 mg* Attention: Dispense and store Tabrecta - in original container with the desiccant - to protect from moisture. Rx only - 56 Film-coated ...
-
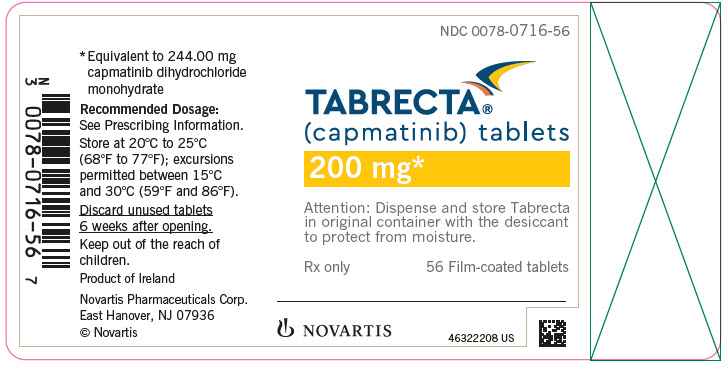
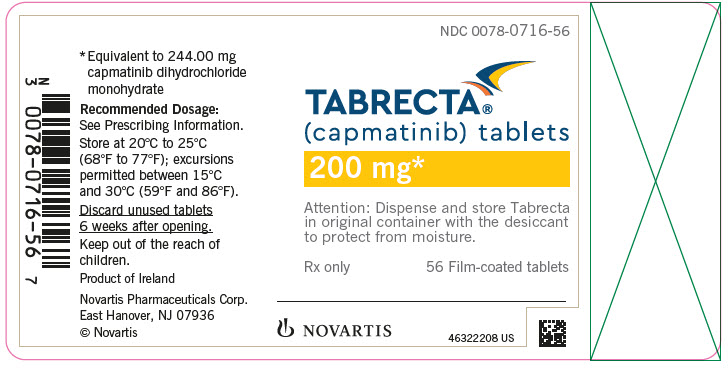
PRINCIPAL DISPLAY PANELNDC 0078-0716-56 - TABRECTA® (capmatinib) tablets - 200 mg* Attention: Dispense and store Tabrecta - in original container with the desiccant - to protect from moisture. Rx only - 56 Film-coated ...
-
INGREDIENTS AND APPEARANCEProduct Information