Label: SYNAGIS- palivizumab injection, solution
- NDC Code(s): 66658-230-01, 66658-231-01
- Packager: Swedish Orphan Biovitrum AB (publ)
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SYNAGIS safely and effectively. See full prescribing information for SYNAGIS. SYNAGIS® (palivizumab) injection, for intramuscular ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Synagis is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients: with a history of premature birth (less than ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information - The recommended dose of Synagis is 15 mg per kg of body weight given monthly by intramuscular injection. The first dose of Synagis should be administered prior to ...
-
3 DOSAGE FORMS AND STRENGTHS
Single-dose liquid solution vials: 50 mg per 0.5 mL and 100 mg per 1 mL.
-
4 CONTRAINDICATIONS
Synagis is contraindicated in children who have had a previous significant hypersensitivity reaction to Synagis [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions - Cases of anaphylaxis and anaphylactic shock, including fatal cases, have been reported following initial exposure or re-exposure to Synagis. Other acute ...
-
6 ADVERSE REACTIONS
The most serious adverse reactions occurring with Synagis are anaphylaxis and other acute hypersensitivity reactions [see Warnings and Precautions (5.1)]. 6.1 Clinical Studies ...
-
7 DRUG INTERACTIONS
No formal drug-drug interaction studies were conducted. In Trial 1, the proportions of children in the placebo and Synagis groups who received routine childhood vaccines, influenza vaccine ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Synagis is not indicated for use in females of reproductive potential. 8.2 Lactation - Risk Summary - Synagis is not indicated for use in females of ...
-
10 OVERDOSAGE
Overdoses with doses up to 85 mg per kg have been reported in clinical studies and post-marketing experience with Synagis, and in some cases, adverse reactions were reported. In case of ...
-
11 DESCRIPTION
Palivizumab is a humanized monoclonal antibody (IgG1κ) produced by recombinant DNA technology, directed to an epitope in the A antigenic site of the F protein of RSV. Palivizumab is a composite of ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Palivizumab is a recombinant humanized monoclonal antibody with anti-RSV activity [see Microbiology (12.4)]. 12.3 Pharmacokinetics - In children less than or equal ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis, mutagenesis, and reproductive toxicity studies have not been performed.
-
14 CLINICAL STUDIES
The safety and efficacy of Synagis were assessed in two randomized, double-blind, placebo-controlled trials of prophylaxis against RSV infection in children at high risk of an RSV-related ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
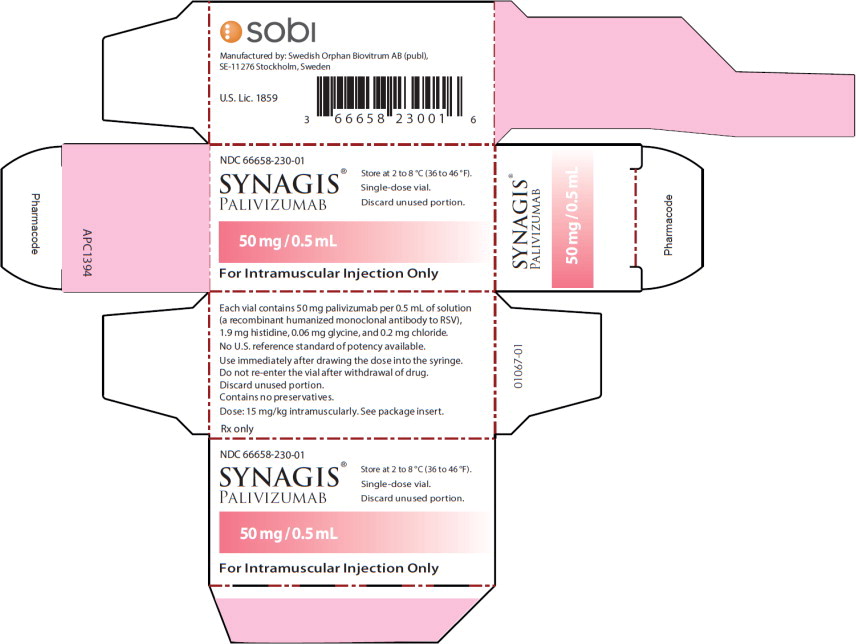
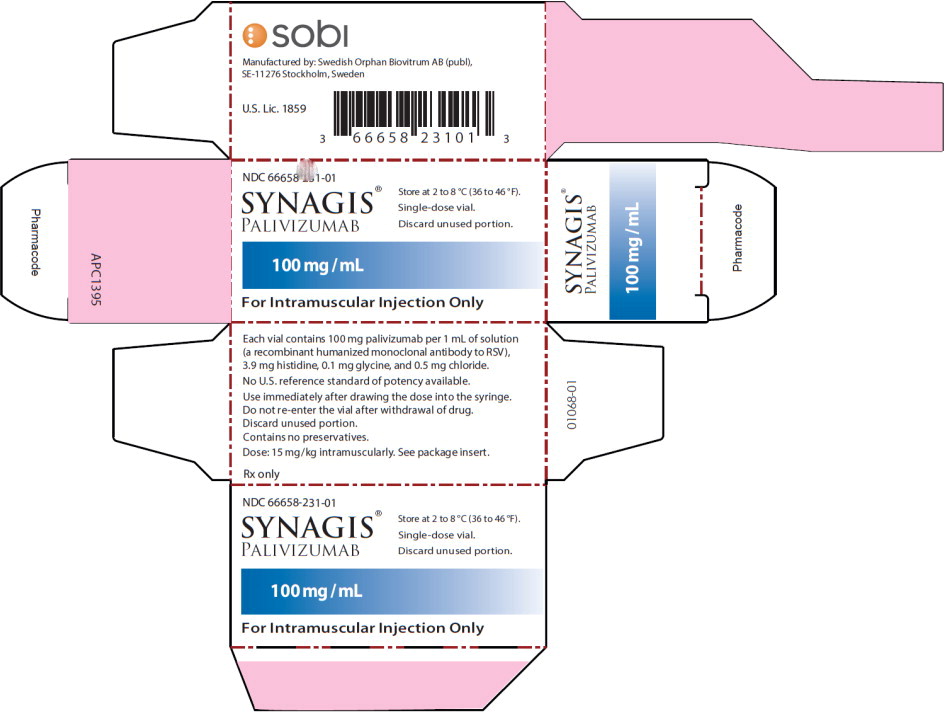
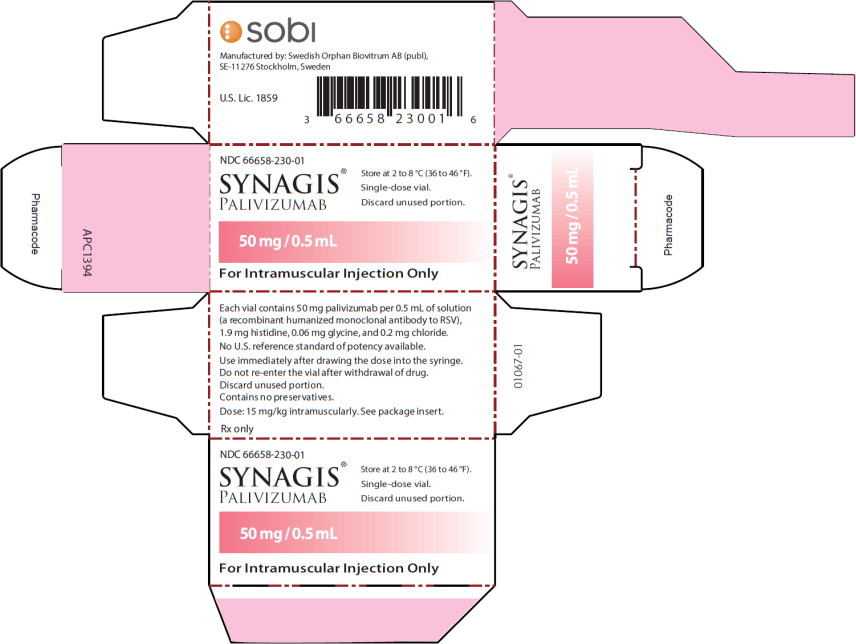
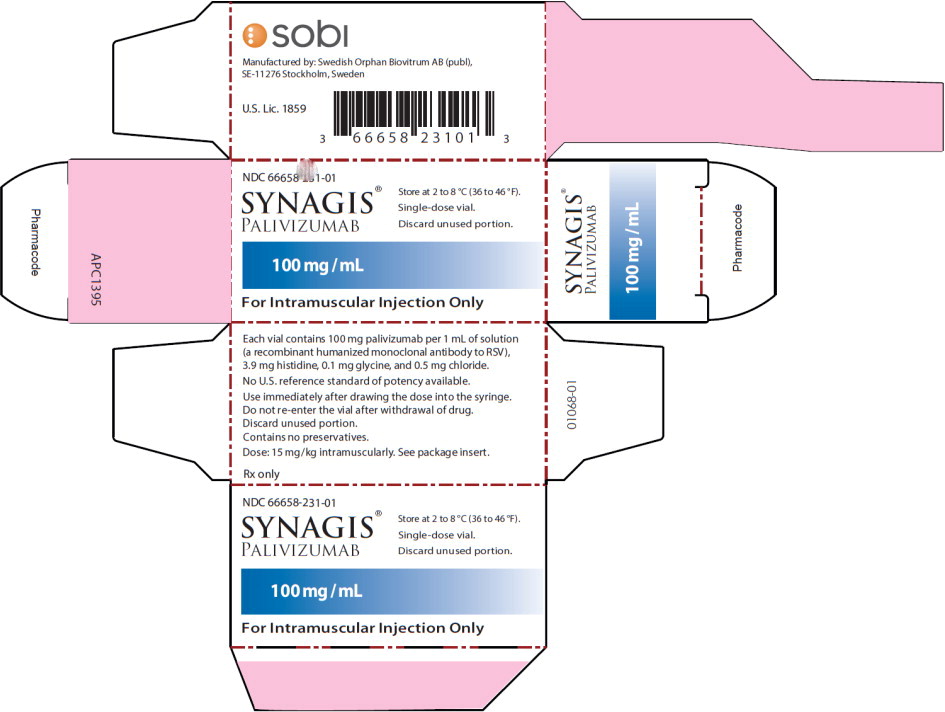
Synagis is supplied in single-dose vials as a preservative-free, sterile liquid solution at 100 mg per mL for intramuscular injection. 50 mg vial NDC 66658-230-01 - The 50 mg vial contains 50 mg ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient's caregiver to read the FDA-approved patient labeling (Patient Information) Hypersensitivity Reactions - Inform the patient's caregiver of the signs and symptoms of ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration - Revised: 11/2021 - PATIENT INFORMATION - SYNAGIS® (Si-na-jis) (palivizumab) injection - What is ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 50 mg/0.5 mL Carton Label - NDC 66658-230-01 - SYNAGIS® PALIVIZUMAB - 50 mg/0.5 mL - For Intramuscular Injection Only - Store at 2 to 8°C (36 to 46°F). Single-dose ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 50 mg/0.5 mL Vial Label - NDC 66658-230-01 - 50 mg/0.5 mL - SYNAGIS® PALIVIZUMAB - For Intramuscular - Injection Only - US Lic. 1859
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 100 mg/mL Carton Label - NDC 66658-231-01 - SYNAGIS® PALIVIZUMAB - 100 mg/mL - For Intramuscular Injection Only - Store at 2 to 8°C (36 to 46°F). Single-dose ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 100 mg/mL Vial Label - NDC 66658-231-01 - 100 mg/mL - SYNAGIS® PALIVIZUMAB - For Intramuscular - Injection Only - US Lic. 1859
-
INGREDIENTS AND APPEARANCEProduct Information