Label: SYMPROIC- naldemedine tablet
- NDC Code(s): 59385-041-07, 59385-041-30
- Packager: BioDelivery Sciences International Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SYMPROIC safely and effectively. See full prescribing information for SYMPROIC. SYMPROIC® (naldemedine tablets for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESYMPROIC is indicated for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration - Alteration of analgesic dosing regimen prior to initiating SYMPROIC is not required. Patients receiving opioids for less than 4 weeks may be less responsive to SYMPROIC [see ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 0.2 mg naldemedine; supplied as yellow, round, film-coated, debossed with Shionogi marking above the identifier code 222 on one side and 0.2 on the other side.
-
4 CONTRAINDICATIONSSYMPROIC is contraindicated in: Patients with known or suspected gastrointestinal obstruction and patients at increased risk of recurrent obstruction, due to the potential for gastrointestinal ...
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Perforation - Cases of gastrointestinal perforation have been reported with use of another peripherally acting opioid antagonist in patients with conditions that may be ...
-
6 ADVERSE REACTIONSSerious and important adverse reactions described elsewhere in labeling include: Gastrointestinal perforation [see Warnings and Precautions (5.1)] Opioid withdrawal [see Warnings and Precautions ...
-
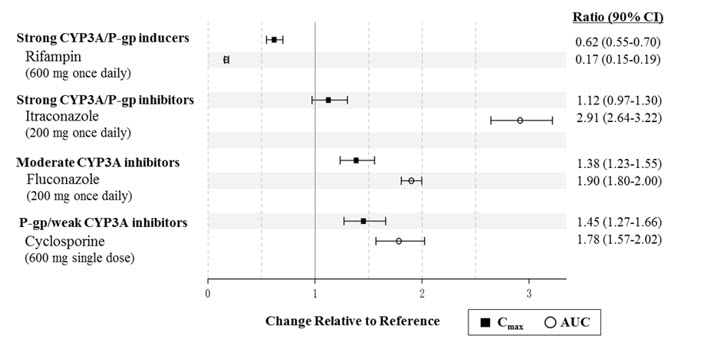
7 DRUG INTERACTIONSTable 3 includes drugs with clinically important drug interactions with SYMPROIC and instructions for preventing or managing the interaction. Table 3: Clinically Relevant Interactions Affecting ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with naldemedine in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. There is a potential for ...
-
8.6 Hepatic ImpairmentThe effect of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of naldemedine has not been evaluated. Avoid use of SYMPROIC in patients with severe hepatic impairment. No ...
-
10 OVERDOSAGESingle doses of naldemedine up to 100 mg (500 times the recommended dose) and multiple doses of up to 30 mg (150 times the recommended dose) for 10 days have been administered to healthy subjects ...
-
11 DESCRIPTIONSYMPROIC (naldemedine), an opioid antagonist, contains naldemedine tosylate as the active ingredient. The chemical name for naldemedine tosylate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Naldemedine is an opioid antagonist with binding affinities for mu-, delta-, and kappa-opioid receptors. Naldemedine functions as a peripherally-acting mu-opioid ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In 2-year carcinogenicity studies, there were no drug-related neoplastic findings following oral administration of ...
-
14 CLINICAL STUDIESSYMPROIC was evaluated in two replicate, 12-week, randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in which SYMPROIC was used without laxatives in patients with OIC and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSYMPROIC is supplied as 0.2 mg naldemedine tablets as follows: bottle of 30 tablets - NDC 59385-041-30 - Store SYMPROIC in a light resistant container at 20°C to 25°C (68°F to 77°F); excursions ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Administration - Advise patients to discontinue SYMPROIC if treatment with the opioid pain medication is also ...
-
SPL UNCLASSIFIED SECTIONSYMPROIC is a registered trademark of Shionogi & Co., Ltd. Manufactured for: BioDelivery Sciences International, Inc. Raleigh, NC 27612 - SYM-001-PI-MAY2020
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: May 2020 - MEDICATION GUIDE - SYMPROIC® (sim proe' ik) (naldemedine) tablets, for oral use - What ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Tablet Bottle Label - 30 TabletsNDC 59385-041-30 - Rx ONLY - 30 Tablets - Symproic® (naldemedine) tablets - 0.2 mg - Dispense the enclosed Medication Guide - to each patient. biodelivery - SCIENCES - © 2019 BioDelivery Sciences ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Tablet Blister Pack7 Tablets - Each tablet contains 0.2 mg of naldemedine - (equivalent to 0.26 mg of naldemedine tosylate). Usual adult dosage: See package insert. Store at 20°C to 25°C (68°F to ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Tablet Blister Pack CartonNDC 59385-041-07 - 7 Tablets - Dosage: Take 1 tablet - once daily - Each tablet contains 0.2 mg of - naldemedine (equivalent to - 0.26 mg of naldemedine tosylate). Please see the ...
-
INGREDIENTS AND APPEARANCEProduct Information