Label: SYMPAZAN- clobazam film
- NDC Code(s): 10094-205-01, 10094-205-60, 10094-210-01, 10094-210-60, view more
- Packager: Aquestive Therapeutics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated March 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SYMPAZAN® safely and effectively. See full prescribing information for SYMPAZAN®. SYMPAZAN® (clobazam) oral film, CIV - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), and Drug Interactions (7.1)].
- The use of benzodiazepines, including SYMPAZAN, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing SYMPAZAN and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines, including SYMPAZAN, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of SYMPAZAN after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue SYMPAZAN or reduce the dosage [see Dosage and Administration (2.2) and Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGESYMPAZAN® is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome (LGS) in patients 2 years of age or older.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - A daily dose of SYMPAZAN® greater than 5 mg should be administered in divided doses twice daily; a 5 mg daily dose can be administered as a single dose. Dose patients ...
-
3 DOSAGE FORMS AND STRENGTHSSYMPAZAN® Oral Film: Thin, white, rectangular, orally dissolving film strips: 5 mg imprinted with C5 - 10 mg imprinted with C10 - 20 mg imprinted with C20
-
4 CONTRAINDICATIONSSYMPAZAN® is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients. Hypersensitivity reactions have included serious dermatological reactions [see Warnings ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks from Concomitant Use with Opioids - Concomitant use of benzodiazepines, including SYMPAZAN®, and opioids may result in profound sedation, respiratory depression, coma, and death ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include the following: Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)] Abuse ...
-
7 DRUG INTERACTIONS7.1 Opioids - The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as SYMPAZAN®, during pregnancy. Healthcare ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - SYMPAZAN® contains clobazam, a Schedule IV controlled substance. 9.2 Abuse - SYMPAZAN is a benzodiazepine and a CNS depressant with a potential for abuse and ...
-
10 OVERDOSAGEOverdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion ...
-
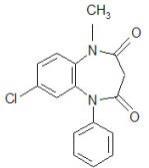
11 DESCRIPTIONSYMPAZAN® contains clobazam, a benzodiazepine derivative, which is chemically known as 7-Chloro-1-methyl-5-phenyl-1H-1,5 benzodiazepine-2,4(3H,5H)-dione with a molecular formula of C16H13ClN2O2 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The exact mechanism of action for clobazam, a 1,5-benzodiazepine, is not fully understood but is thought to involve potentiation of GABAergic neurotransmission ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In mice, oral administration of clobazam (0, 6, 12, or 24 mg/kg/day) for 2 years did not result in an increase in ...
-
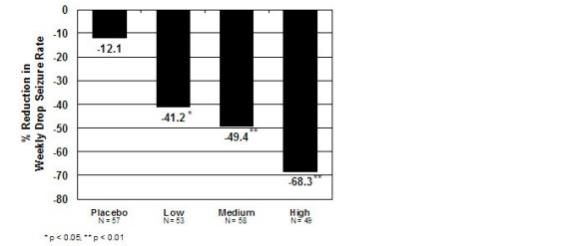
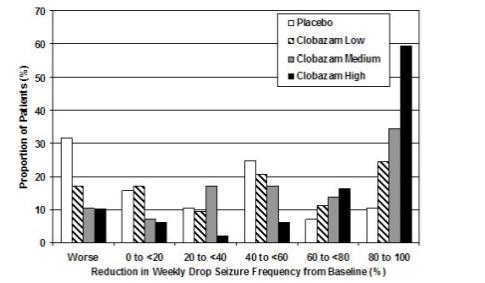
14 CLINICAL STUDIES14.1 Demonstration of Pharmacokinetic Equivalence Between SYMPAZAN® and Clobazam Tablets - The efficacy of SYMPAZAN® is based upon bioavailability studies comparing clobazam tablets to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach SYMPAZAN® oral film is a white rectangular film that contains 5 mg, 10 mg or 20 mg of clobazam and printed in black ink either "C5,” "C10" or "C20" on the strip according to their respective ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Risks from Concomitant Use with Opioids - Inform patients and caregivers that potentially ...
-
MEDICATION GUIDEMEDICATION GUIDE - SYMPAZAN®(SYM-pa-zan) (clobazam) oral film, CIV - What is the most important information I should know about SYMPAZAN? SYMPAZAN is a benzodiazepine medicine ...
-
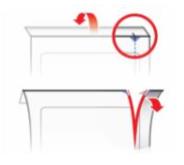
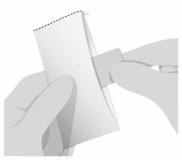
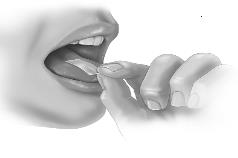
PATIENT PACKAGE INSERTInstructions for Use - SYMPAZAN® (SIM-pa-zan) (clobazam) oral film, CIV - Read this Instructions for Use before you start using SYMPAZAN and each time you get a refill. There may be new ...
-
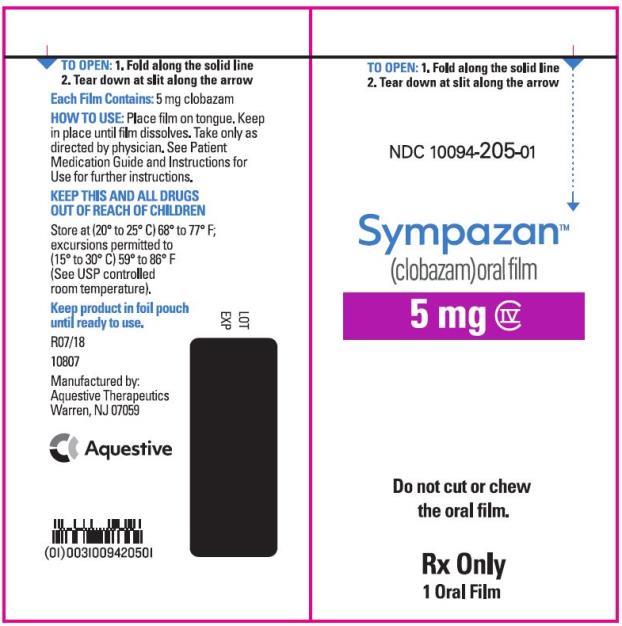
PRINCIPAL DISPLAY PANEL
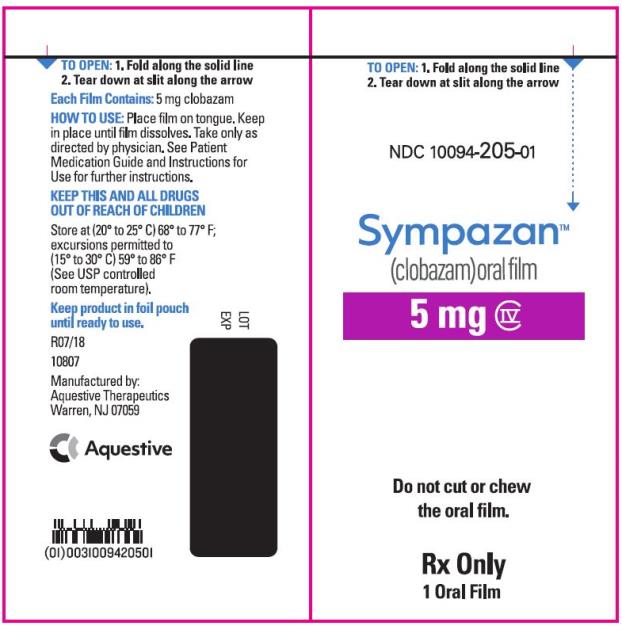
NDC 10094-205-01 - Sympazan - (clobazam) Oral film - 5 mg - Rx Only - 1 Oral films
-
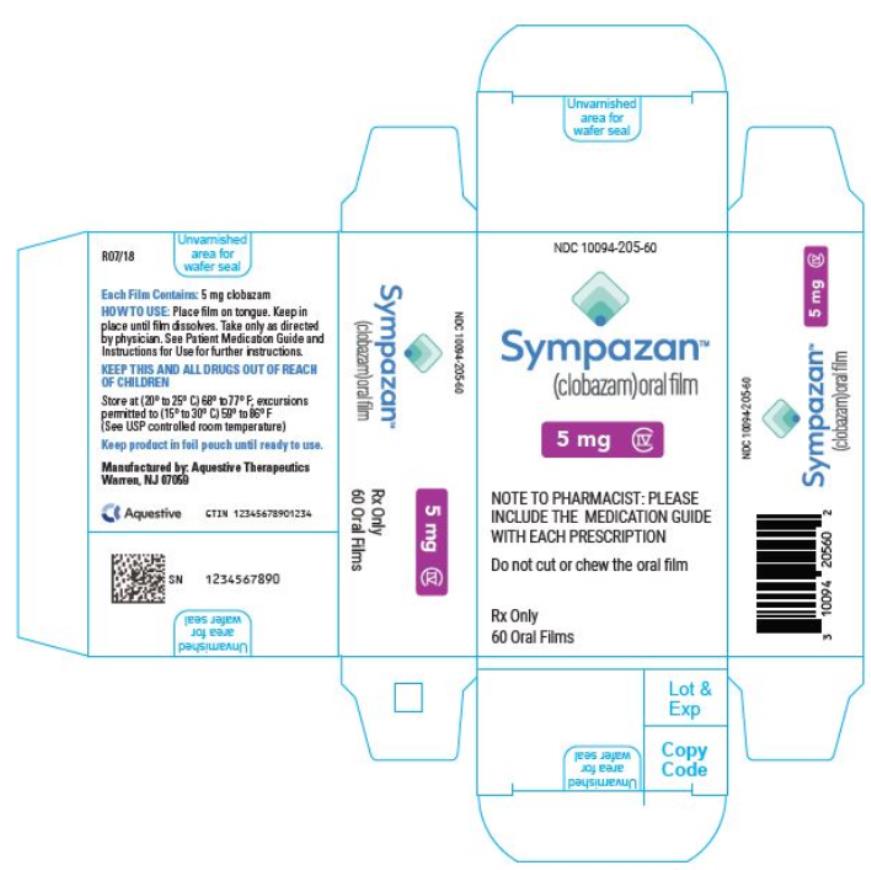
PRINCIPAL DISPLAY PANEL
NDC 10094-205-60 - Sympazan - (clobazam) Oral film - 5 mg - Rx Only - 60 Oral films
-
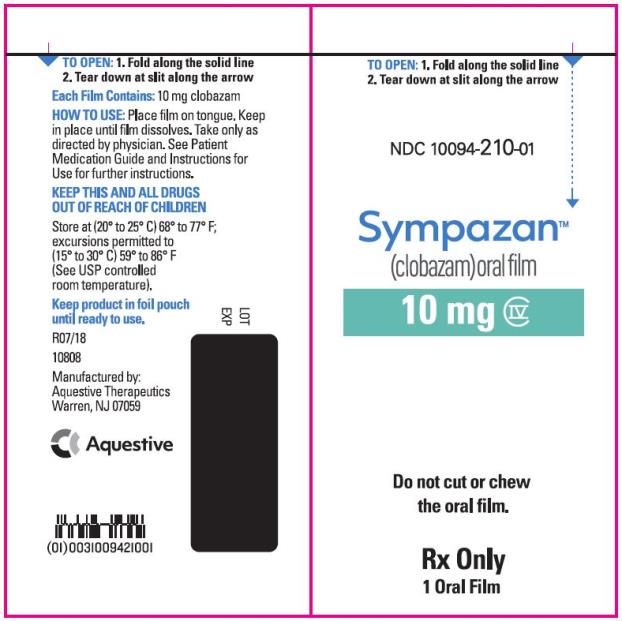
PRINCIPAL DISPLAY PANEL
NDC 10094-210-01 - Sympazan - (clobazam) Oral film - 10 mg - Rx Only - 1 Oral films
-
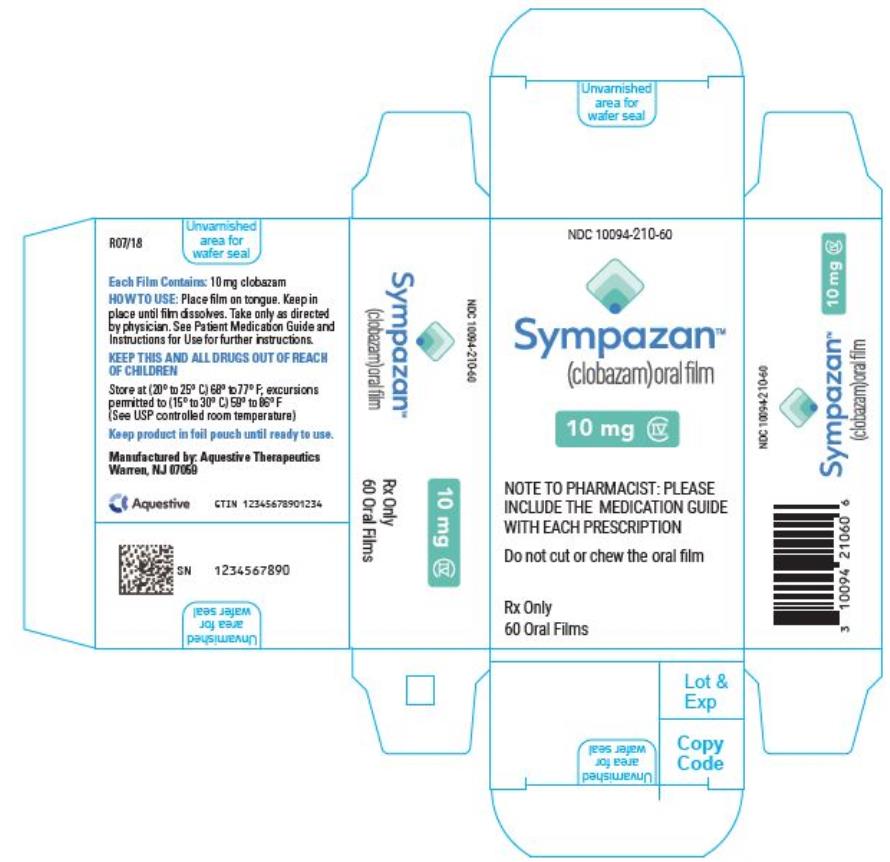
PRINCIPAL DISPLAY PANEL
NDC 10094-210-60 - Sympazan - (clobazam) Oral film - 10 mg - Rx Only - 60 Oral films
-
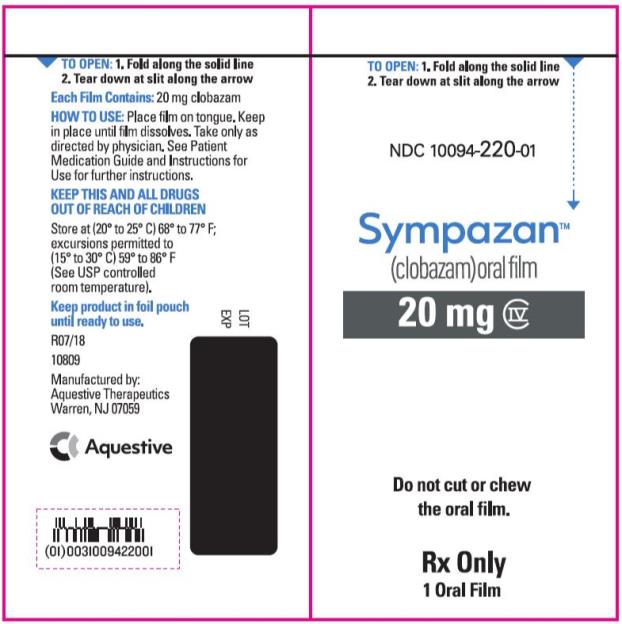
PRINCIPAL DISPLAY PANEL
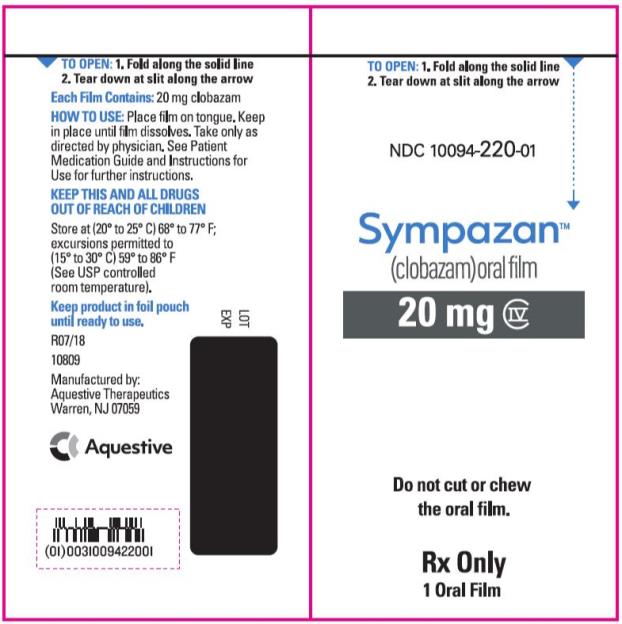
NDC 10094-220-01 - Sympazan - (clobazam) Oral film - 20 mg - Rx Only - 1 Oral films
-
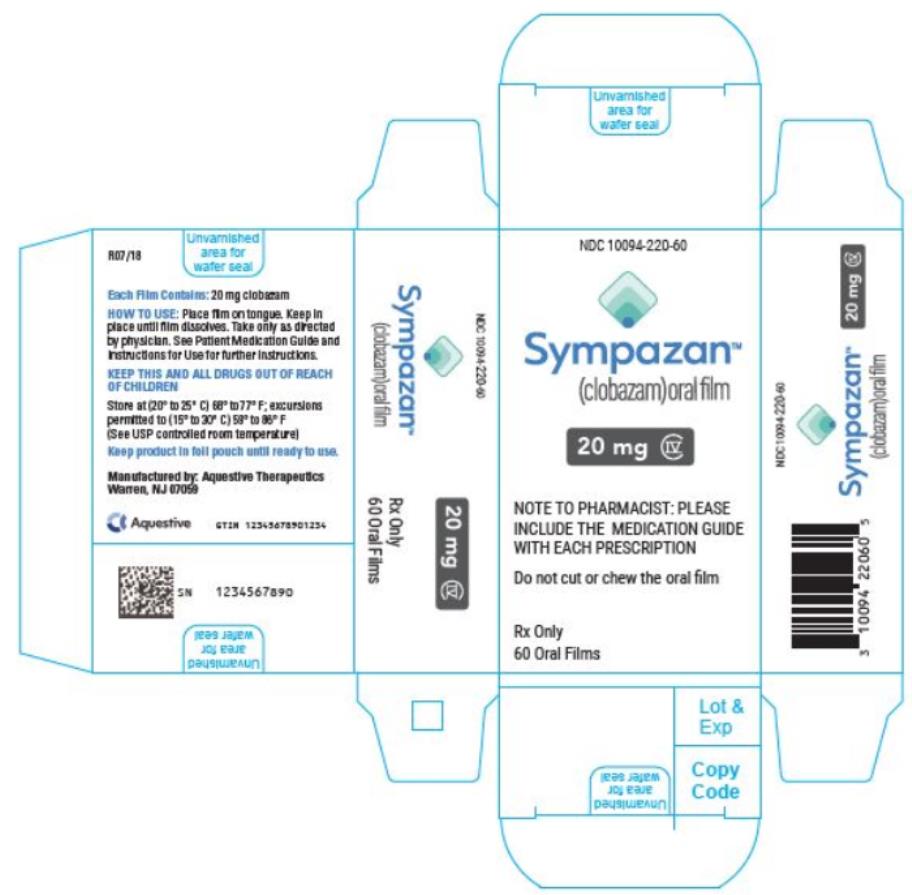
PRINCIPAL DISPLAY PANEL
NDC 10094-220-60 - Sympazan - (clobazam) Oral film - 20 mg - Rx Only - 60 Oral films
-
INGREDIENTS AND APPEARANCEProduct Information