Label: SUCRAID- sacrosidase solution
- NDC Code(s): 67871-111-01, 67871-111-04, 67871-111-05, 67871-111-06, view more
- Packager: QOL Medical, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONSacrosidase is an enzyme with the chemical name of β,D-fructofuranoside fructohydrolase. The enzyme is derived from baker’s yeast (Saccharomyces cerevisiae). It has been reported that the primary ...
-
CLINICAL PHARMACOLOGYCongenital sucrase-isomaltase deficiency (CSID) is a chronic, autosomal recessive, inherited, phenotypically heterogeneous disease with very variable enzyme activity. CSID is usually characterized ...
-
CLINICAL STUDIESA two-phase (dose response preceded by a breath hydrogen phase) double-blind, multi-site, crossover trial was conducted in 28 pediatric patients (approximately 5 months to 12 years of age) with ...
-
INDICATIONS AND USAGESucraid® (sacrosidase) Oral Solution is indicated for the treatment of sucrase deficiency, which is part of congenital sucrase-isomaltase deficiency (CSID), in adult and pediatric patients 5 ...
-
CONTRAINDICATIONSSucraid is contraindicated in patients known to be hypersensitive to yeast, yeast products, glycerin (glycerol), or papain (see WARNINGS).
-
WARNINGSSevere Hypersensitivity Reactions - Severe hypersensitivity reactions, including wheezing, rash, and pruritis, have been reported with administration of Sucraid. Sucraid contains papain, which is ...
-
PRECAUTIONSIncreased Blood Glucose Concentrations in Patients with Diabetes Mellitus - Sucraid enables the products of sucrose hydrolysis, glucose and fructose, to be absorbed and may increase blood glucose ...
-
ADVERSE REACTIONSThe following adverse reactions associated with the use of sacrosidase were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from ...
-
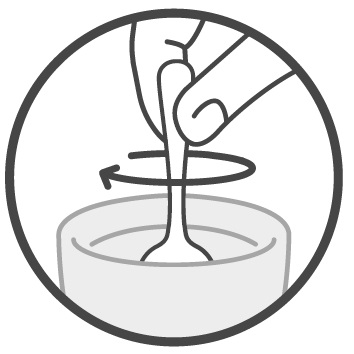
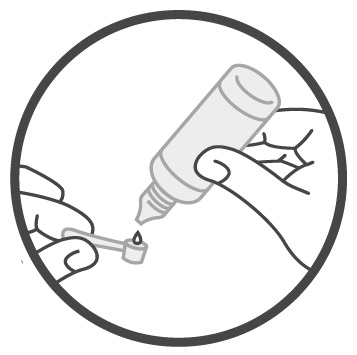
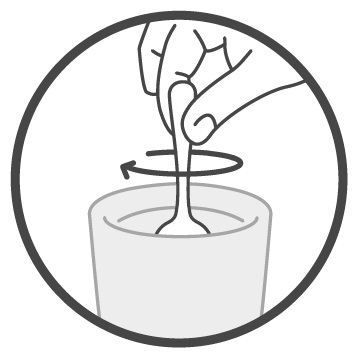
DOSAGE AND ADMINISTRATIONImportant Administration Information - Administer Sucraid with each meal or snack. Mix Sucraid with cold or room temperature water, milk or infant formula prior to administration. Administration ...
-
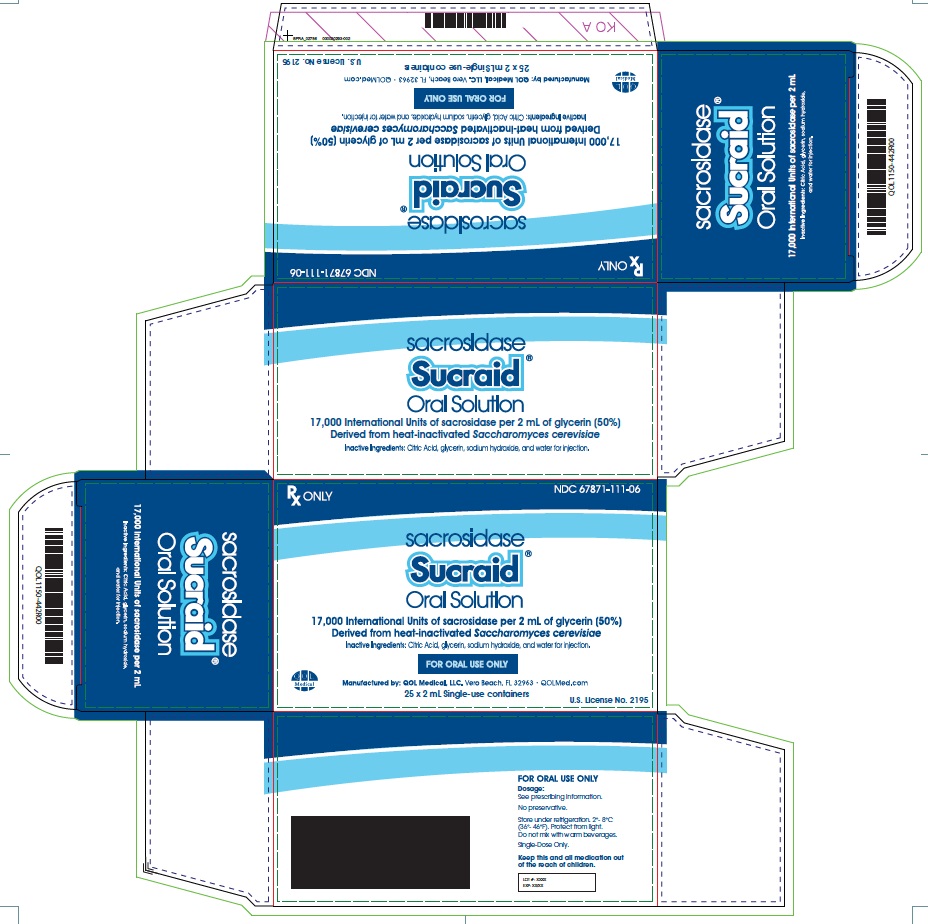
HOW SUPPLIED118 mL Multiple-Dose Bottle - Sucraid (sacrosidase) Oral Solution is available in 118 mL (4 fluid ounces) multiple-dose translucent plastic bottles, packaged two bottles per carton. Each mL of ...
-
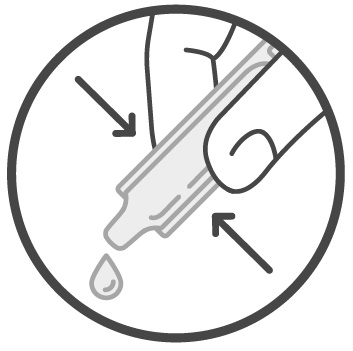
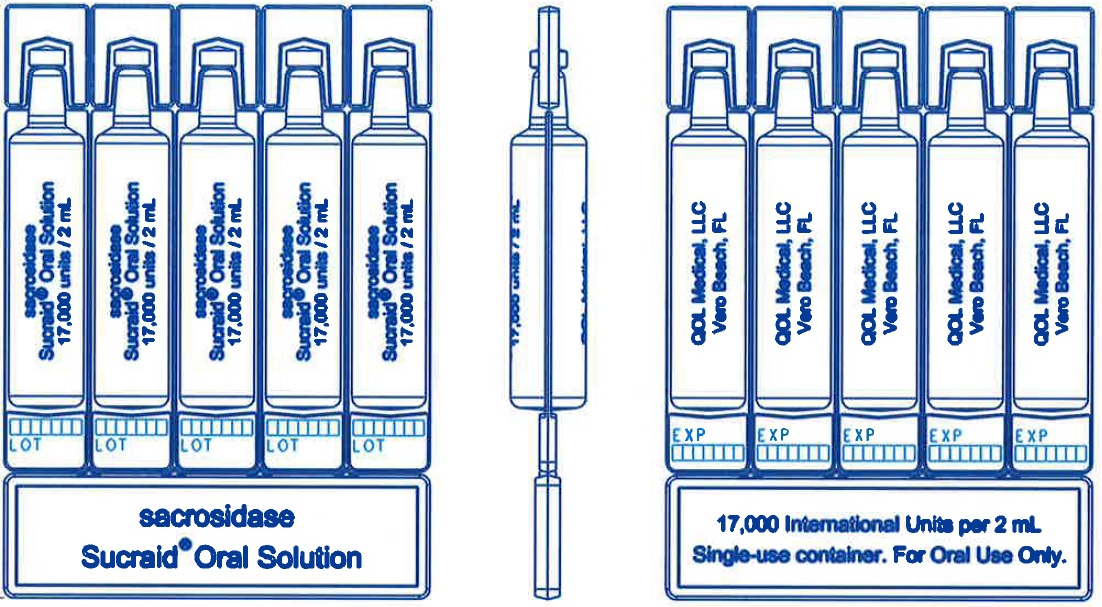
Instructions for UseSucraid® (Su-kreid) (sacrosidase) Oral Solution: 2-mL Single-Use Container - Read this Instructions for Use before you start taking or giving Sucraid to a child, and each time you get a refill ...
-
Instructions for UseSUCRAID® (Su-kreid) (sacrosidase) oral solution - 118 mL Multiple-Dose Bottle - Read this Instructions for Use before you start taking or giving SUCRAID to a child, and each time you get a refill ...
-
INFORMATION FOR PATIENTSPatient Information - SUCRAID® (Su-kreid) (sacrosidase) Oral Solution - What is SUCRAID? SUCRAID is a prescription medicine for the treatment of people who were born with a lack of ...
-
118 mL Multi-dose bottleCarton:
-
PRINCIPAL DISPLAY PANELBottle label:
-
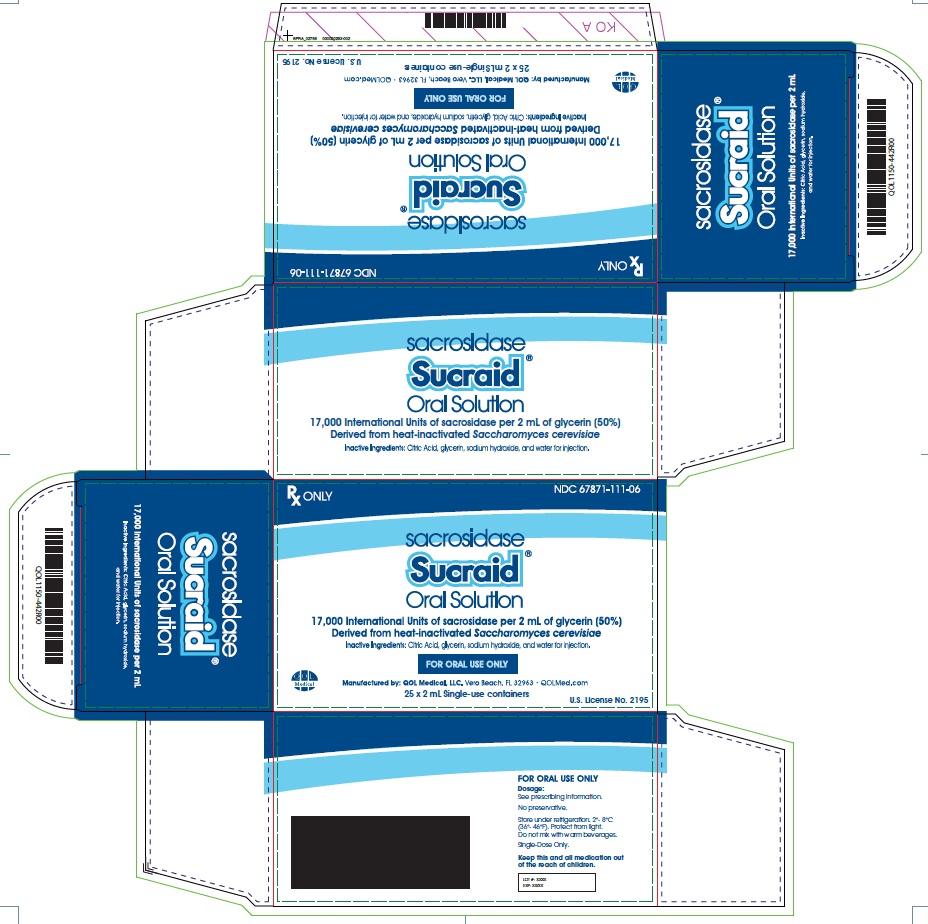
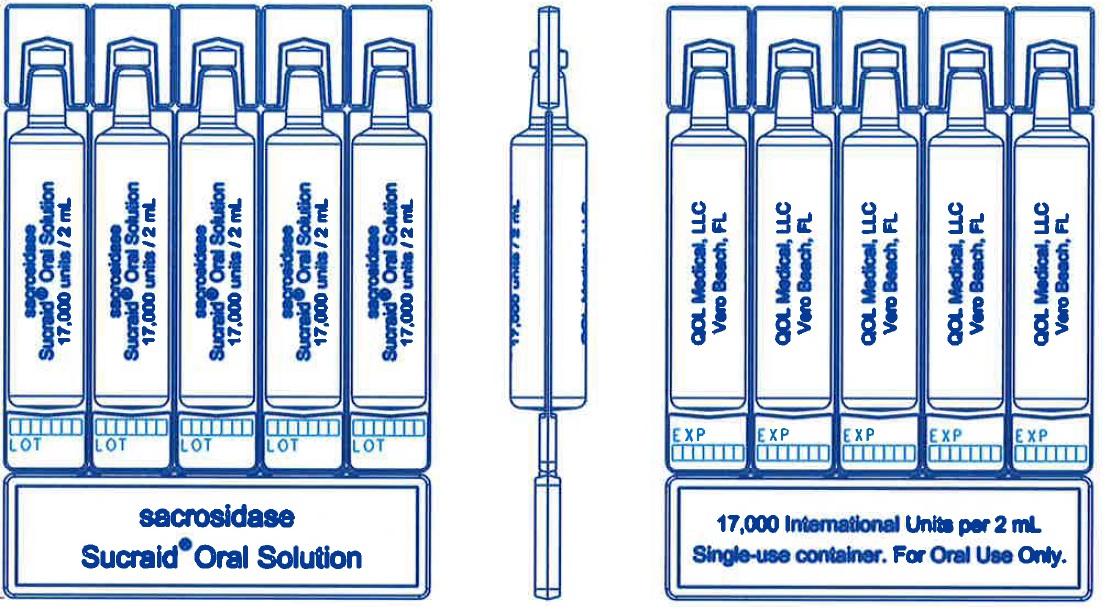
2 mL Single-use containerCarton: Box: Pouch: Single-use container:
-
INGREDIENTS AND APPEARANCEProduct Information