Label: STRENSIQ- asfotase alfa solution
- NDC Code(s): 25682-010-01, 25682-010-12, 25682-013-01, 25682-013-12, view more
- Packager: Alexion Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use STRENSIQ safely and effectively. See full prescribing information for STRENSIQ. STRENSIQ® (asfotase alfa) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate STRENSIQ under the supervision of a healthcare provider with appropriate medical monitoring and support measures. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue STRENSIQ and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGESTRENSIQ® is indicated for the treatment of patients with perinatal/infantile- and juvenile-onset hypophosphatasia (HPP).
-
2 DOSAGE AND ADMINISTRATION2.1 Recommendations Prior to STRENSIQ Treatment - Initiate STRENSIQ under the supervision of a healthcare provider with appropriate medical monitoring and support measures [see Warnings and ...
-
3 DOSAGE FORMS AND STRENGTHSSTRENSIQ is a clear, slightly opalescent or opalescent, colorless to slightly yellow aqueous solution; few small translucent or white particles may be present. The product is available ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions Including Anaphylaxis - Life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with enzyme replacement ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described below and elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Lipodystrophy [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drug Interference with Laboratory Tests - Laboratory Tests Utilizing Alkaline Phosphatase as a Detection Reagent - Studies have shown that there is analytical interference between ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on STRENSIQ use in pregnant women to inform a drug associated risk. In animal reproduction studies, asfotase alfa administered ...
-
11 DESCRIPTIONAsfotase alfa is a tissue nonspecific alkaline phosphatase (TNSALP) produced by recombinant DNA technology in a Chinese hamster ovary cell line. Asfotase alfa is a soluble glycoprotein composed of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - HPP is caused by a deficiency in TNSALP enzyme activity, which leads to elevations in several TNSALP substrates, including inorganic pyrophosphate (PPi). TNSALP is a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with ...
-
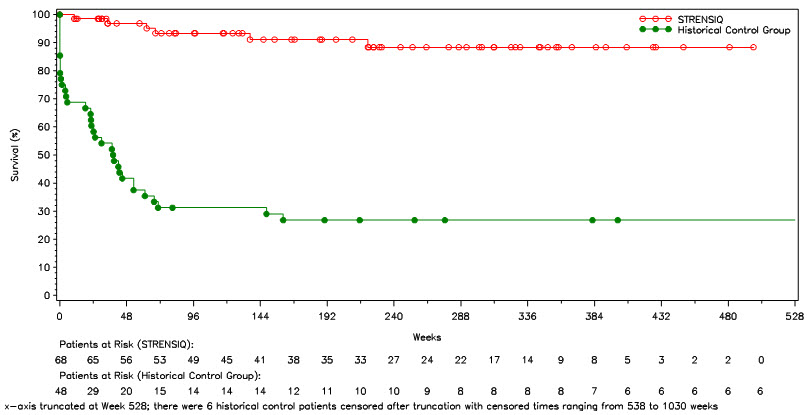
14 CLINICAL STUDIES14.1 Perinatal/Infantile-Onset HPP - Study 1 was a 24-week prospective single-arm trial in 11 patients with severe perinatal/infantile-onset HPP. In this study, 7/11 (64%) were female and 10/11 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSTRENSIQ is supplied as a sterile, nonpyrogenic, preservative-free, clear, slightly opalescent or opalescent, colorless to slightly yellow aqueous solution; a few small translucent or white ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Advise patients or caregivers of the following: Preparation - When ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Alexion Pharmaceuticals, Inc. 121 Seaport Boulevard - Boston, MA 02210 USA - U.S. License Number: 1743 - STRENSIQ is a trademark of Alexion Pharmaceuticals, Inc. © 2024 Alexion ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 7/2024 - PATIENT INFORMATION - STRENSIQ® [stren sik] (asfotase alfa) injection, for ...
-
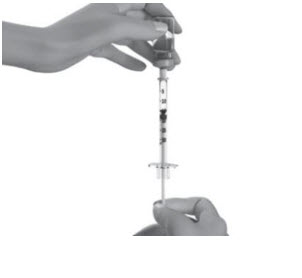
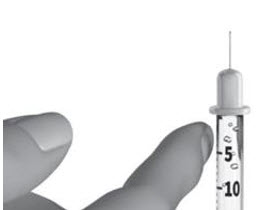
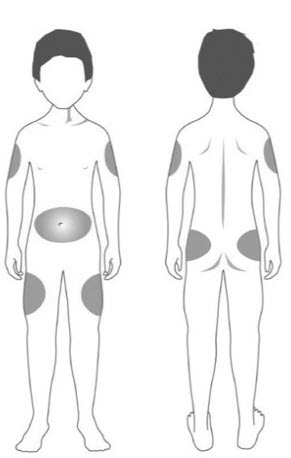
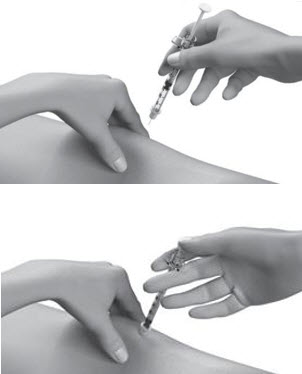
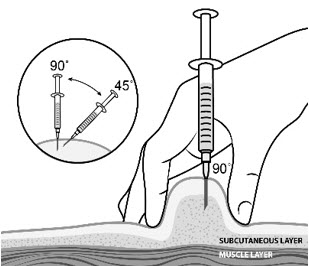
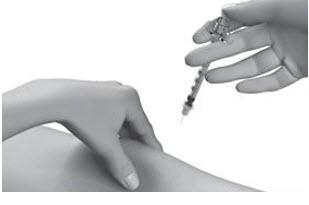
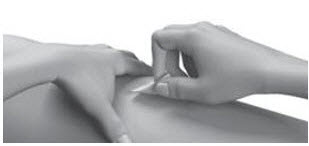
INSTRUCTIONS FOR USESTRENSIQ® [stren' sik](asfotase alfa)injection, for subcutaneous usevialRead this "Instructions for Use" before you start using STRENSIQ and each time you get a refill. There may be new information. This information does not take the place of talking to your ...
-
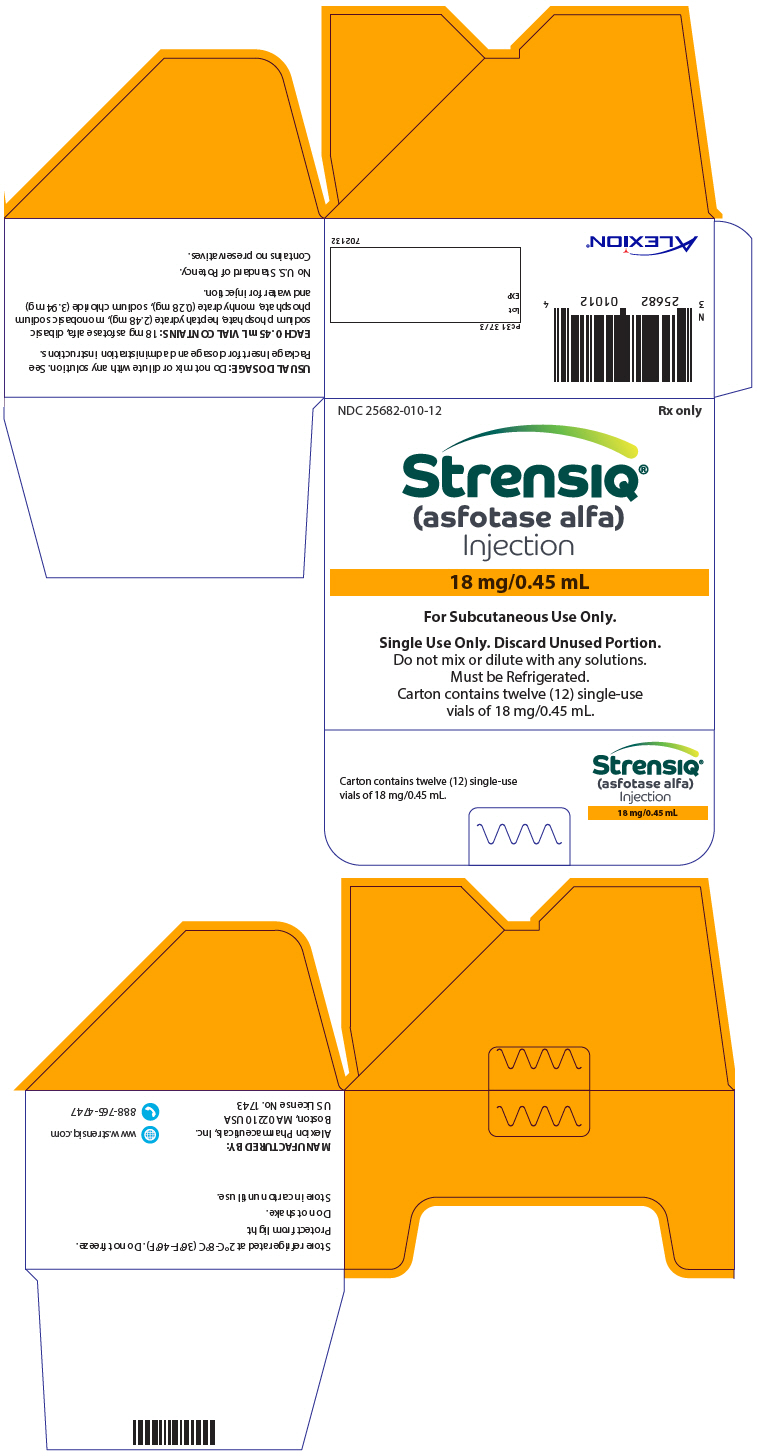
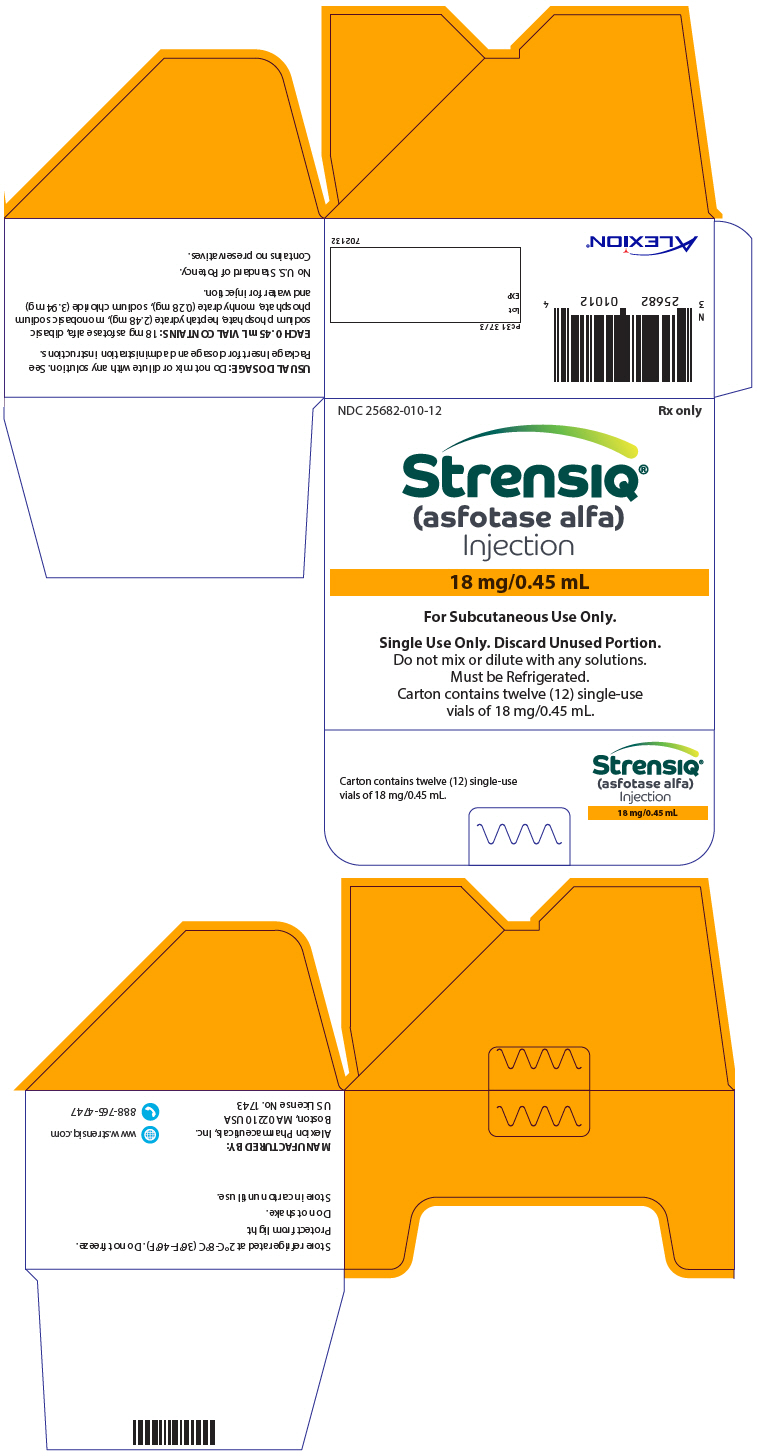
PRINCIPAL DISPLAY PANEL - 18 mg/0.45 mL Vial CartonNDC 25682-010-12 - Rx only - STRENSIQ® (asfotase alfa) Injection - 18 mg/0.45 mL - For Subcutaneous Use Only. Single Use Only. Discard Unused Portion. Do not mix or dilute with any solutions. Must be ...
-
PRINCIPAL DISPLAY PANEL - 28 mg/0.7 mL Vial CartonNDC 25682-013-12 - Rx only - STRENSIQ® (asfotase alfa) Injection - 28 mg/0.7 mL - For Subcutaneous Use Only. Single Use Only. Discard Unused Portion. Do not mix or dilute with any solutions. Must be ...
-
PRINCIPAL DISPLAY PANEL - 40 mg/mL Vial CartonNDC 25682-016-12 - Rx only - STRENSIQ® (asfotase alfa) Injection - 40 mg/mL - For Subcutaneous Use Only. Single Use Only. Discard Unused Portion. Do not mix or dilute with any solutions. Must be ...
-
PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Vial CartonNDC 25682-019-12 - Rx only - STRENSIQ® (asfotase alfa) Injection - 80 mg/0.8 mL - For Subcutaneous Use Only. For patients 40 kg and greater. Single Use Only. Discard Unused Portion. Do not mix or dilute ...
-
INGREDIENTS AND APPEARANCEProduct Information