Label: SOTYLIZE- sotalol hydrochloride solution
- NDC Code(s): 24338-530-25, 24338-530-48
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOTYLIZE® safely and effectively. See full prescribing information for SOTYLIZE. SOTYLIZE (sotalol hydrochloride) oral solution ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LIFE-THREATENING PROARRHYTHMIA
To minimize the risk of drug-induced arrhythmia, initiate or re-initiate oral sotalol in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring.
Sotalol can cause life-threatening ventricular tachycardia associated with QT interval prolongation.
If the QT interval prolongs to 500 msec or greater, reduce the dose, lengthen the dosing interval, or discontinue the drug.
Calculate creatinine clearance to determine appropriate dosing [see Dosage and Administration (2.5)].
Close -
1 INDICATIONS AND USAGE1.1 Life-Threatening Ventricular Arrhythmia - SOTYLIZE is indicated for the treatment of documented, life-threatening ventricular arrhythmias, such as sustained ventricular ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Safety Measures of Oral Sotalol Therapy - Withdraw other antiarrhythmic therapy before starting SOTYLIZE and monitor for a minimum of 2 to 3 plasma half-lives prior to initiating ...
-
3 DOSAGE FORMS AND STRENGTHSOral solution: 5 mg/mL, in 250 mL or 480 mL bottles.
-
4 CONTRAINDICATIONSFor the treatment of AFIB/AFL or ventricular arrhythmias, SOTYLIZE is contraindicated in patients with: Baseline QT interval ˃450 msec - Sinus bradycardia, sick sinus syndrome, second and third ...
-
5 WARNINGS AND PRECAUTIONS5.1 QT Prolongation and Proarrhythmia - SOTYLIZE can cause serious and potentially fatal ventricular arrhythmias such as sustained VT/VF, primarily Torsade de Pointes (TdP) type ventricular ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Antiarrhythmics and Other QT Prolonging Drugs Discontinue Class I or Class III antiarrhythmic agents for at least three half-lives prior to dosing with sotalol. Class Ia antiarrhythmic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Both the untreated underlying condition in pregnancy and the use of sotalol in pregnancy cause adverse outcomes to the mother and fetus/neonate (see Clinical ...
-
10 OVERDOSAGEIntentional or accidental overdosage with sotalol has resulted in death. Symptoms and Treatment of Overdosage: The most common signs to be expected are bradycardia, congestive heart ...
-
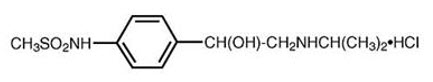
11 DESCRIPTIONSOTYLIZE is an aqueous solution containing sotalol hydrochloride. Sotalol hydrochloride is a white, crystalline solid with a molecular weight of 308.8. It is hydrophilic, soluble in water ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sotalol has both beta-adrenoreceptor blocking (Vaughan Williams Class II) and cardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic ...
-
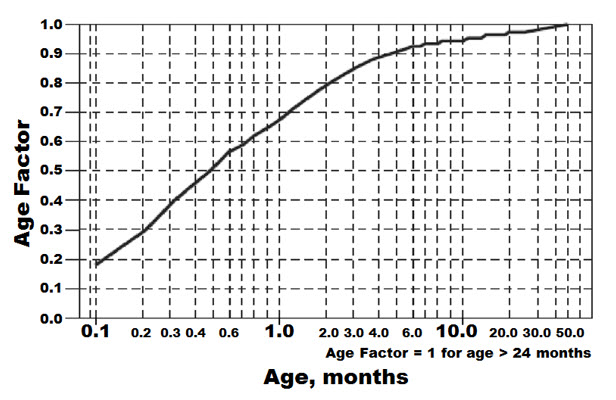
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Calculations of safety margins are for the maximum recommended human dose (MRHD) of 640 mg/day of sotalol, administered for ...
-
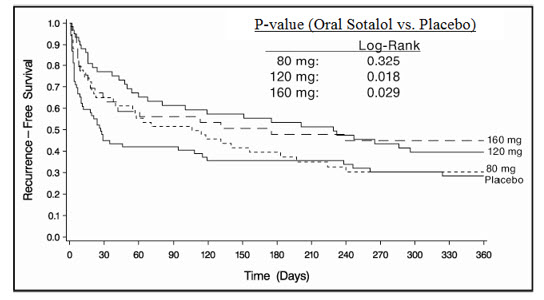
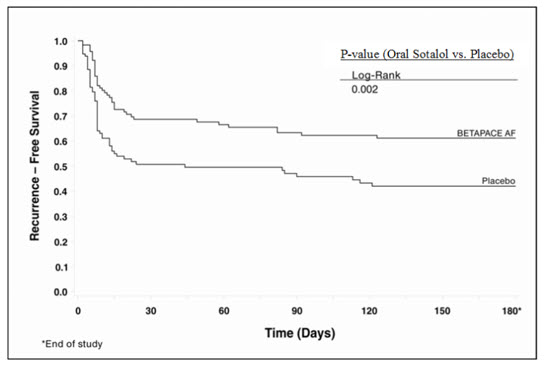
14 CLINICAL STUDIES14.1 Ventricular Arrhythmias - In patients with life-threatening arrhythmias [sustained ventricular tachycardia/fibrillation (VT/VF)], sotalol was studied acutely [by suppression of programmed ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSOTYLIZE (sotalol hydrochloride) is supplied as follows: NDC 24338-530-25, 5 mg/mL: 250 mL bottle - NDC 24338-530-48, 5 mg/mL: 480 mL bottle - Store at 20°C to 25°C (68°F -77°F); excursions ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to contact their healthcare provider in the event of syncope, pre-syncopal symptoms and cardiac palpitations. Advise patients to contact their healthcare provider in the event of ...
-
SPL UNCLASSIFIED SECTIONRx Only - Manufactured for: azurity® pharmaceuticals - Woburn, MA 01801 - Manufactured by: Patheon Inc. Whitby Region Operations - 111 Consumers Drive - Whitby, ON L1N 5Z5 Canada - Patent ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bottle LabelNDC: 24338-530-25 - Sotylize® (sotalol hydrochloride) oral solution - 5 mg/mL - For Oral Use Only - 250 mL - Distributed by: azurity® pharmaceuticals - Woburn, MA 01801 USA
-
INGREDIENTS AND APPEARANCEProduct Information