Label: SORILUX- calcipotriene aerosol, foam
- NDC Code(s): 51862-376-12, 51862-376-60
- Packager: Mayne Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SORILUX safely and effectively. See full prescribing information for SORILUX. SORILUX (calcipotriene) foam, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESORILUX Foam is indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONSORILUX Foam is for topical use only. SORILUX Foam is not for oral, ophthalmic, or intravaginal use. Apply a thin layer of SORILUX Foam twice daily to the affected areas and rub in gently and ...
-
3 DOSAGE FORMS AND STRENGTHS0.005%, white foam. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle.
-
4 CONTRAINDICATIONSSORILUX Foam should not be used by patients with known hypercalcemia.
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - The propellant in SORILUX Foam is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. 5.2 Effects on Calcium ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Although there are no available data on the drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes in pregnant women ...
-
10 OVERDOSAGETopically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with use of topical calcipotriene [See Warnings and ...
-
11 DESCRIPTIONSORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog, in an aqueous-based emulsion foam vehicle for topical dermatologic use. Chemically, calcipotriene is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Calcipotriene is a synthetic vitamin D3 analog that has a similar receptor binding affinity as natural vitamin D3. However, the exact mechanism of action contributing ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Calcipotriene topically administered to mice for up to 24 months at dose levels of 3, 10, or 30 mcg/kg/day (corresponding to 9, 30, or ...
-
14 CLINICAL STUDIESIn two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 659 subjects with psoriasis were randomized 2:1 to SORILUX Foam or vehicle; subjects applied the ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - SORILUX (calcipotriene) Foam, 0.005%, is supplied as follows: 120 g aluminum canNDC 51862-376-12 - 16.2 Storage and Handling - Store at 20ºC to 25°C (68°F to 77°F) ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform the patient to adhere to the following instructions: Apply SORILUX Foam to the affected skin ...
-
SPL UNCLASSIFIED SECTIONSORILUX is a registered trademark of Mayne Pharma LLC. Distributed by: Mayne Pharma - Raleigh, NC 27609 - 141294
-
PATIENT PACKAGE INSERTPatient Information - SORILUX (SOR-i-lux) (calcipotriene) Foam, 0.005% This Patient Information has been approved by the U.S. Food and Drug Administration.Revised ...
-
Instructions for UseSORILUX (SOR-i-lux)(calcipotriene)Foam, 0.005%Read this Instructions for Use before you start using SORILUX Foam and each time you get a refill. There may be new information. This information does not take the place of talking with your ...
-
PRINCIPAL DISPLAY PANEL - 120 gram Can CartonNDC 51862-376-12 - Sorilux® (calcipotriene) Foam, 0.005% 120 grams - Rx only - For topical use only - STORE UPRIGHT - mayne pharma - Recyclable - Aluminum - Container
-
INGREDIENTS AND APPEARANCEProduct Information


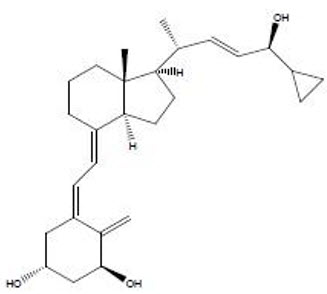
 Figure A
Figure A Figure B
Figure B Figure C
Figure C Figure D
Figure D Figure E
Figure E Figure F
Figure F Figure G
Figure G