Label: SOOLANTRA- ivermectin cream
- NDC Code(s): 0299-3823-00, 0299-3823-02, 0299-3823-30, 0299-3823-45, view more
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOOLANTRA Cream safely and effectively. See full prescribing information for SOOLANTRA Cream. SOOLANTRA - ®(ivermectin) cream, 1% ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESOOLANTRA cream is indicated for the treatment of inflammatory lesions of rosacea.
-
2 DOSAGE AND ADMINISTRATIONApply to the affected areas of the face once daily. Use a pea-size amount for each area of the face (forehead, chin, nose, each cheek) that is affected. Spread as a thin layer, avoiding the eyes ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 1%. Each gram of SOOLANTRA cream contains 10 mg of ivermectin in a white to pale yellow cream base. SOOLANTRA cream is supplied in tubes of 30 g, 45 g and 60 g.
-
4 CONTRAINDICATIONSNone.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSIn vitro studies have shown that SOOLANTRA cream, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data on the use of ivermectin, including SOOLANTRA cream, in pregnant women are insufficient to establish a drug- associated risk of major birth ...
-
10 OVERDOSAGEIn accidental or significant exposure to unknown quantities of veterinary formulations of ivermectin in humans, either by ingestion, inhalation, injection, or exposure to body surfaces, the ...
-
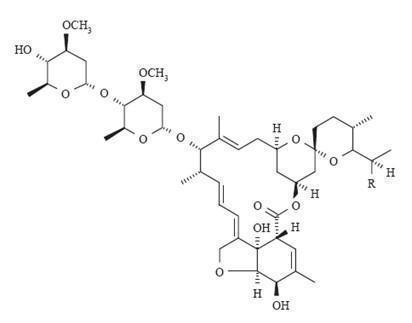
11 DESCRIPTIONSOOLANTRA (ivermectin) cream, 1% is a white to pale yellow hydrophilic cream intended for topical use. Each gram of SOOLANTRA cream contains 10 mg of ivermectin. Ivermectin is a semi-synthetic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of SOOLANTRA cream in treating rosacea lesions is unknown. 12.2 Pharmacodynamics - Cardiac Electrophysiology - At therapeutic doses ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year dermal mouse carcinogenicity study, ivermectin was administered to CD-1 mice at topical doses of 1, 3, and 10 mg/kg/day ...
-
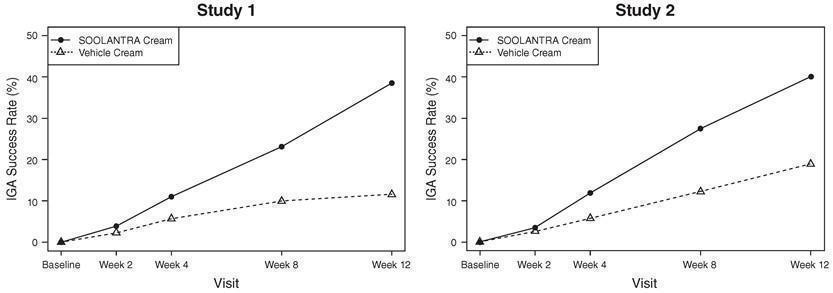
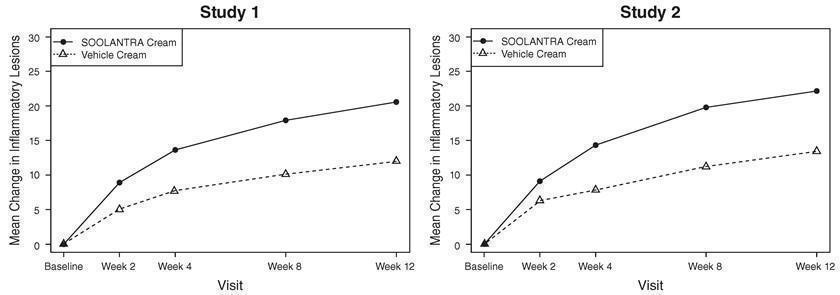
14 CLINICAL STUDIESSOOLANTRA cream applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomized, double-blind, vehicle controlled clinical trials, which were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSOOLANTRA (ivermectin) cream, 1% is a white to pale yellow cream, supplied in a laminated tube with a child resistant cap in the following sizes: 30 gram - NDC0299-3823-30 - 45 gram ...
-
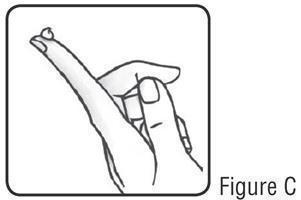
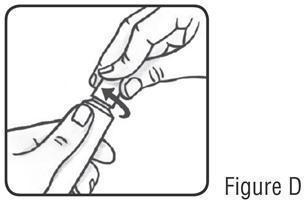
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Patients using SOOLANTRA cream should receive the following instruction: Keep out of reach of ...
-
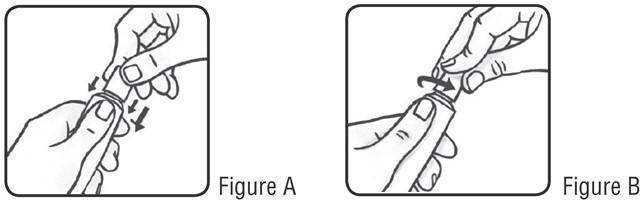
PATIENT INFORMATIONSOOLANTRA® (SOO-LAWN-TRAH) (ivermectin) cream - Important: SOOLANTRA cream is for use on the skin only (topical use).Do not use SOOLANTRA cream in your mouth, eyes, or vagina. What ...
-
Package Label - 45 gNDC 0299-3823-45 - soolantra - ® (ivermectin) cream, 1% NET WT. 45 g - Rx only - For Topical Use Only - Keep Out of Reach of Children - GALDERMA - Marketed by: GALDERMA LABORATORIES ...
-
INGREDIENTS AND APPEARANCEProduct Information