Label: SOLOSEC- secnidazole granule
- NDC Code(s): 27437-051-01, 27437-051-02
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOLOSEC® safely and effectively. See full prescribing information for SOLOSEC. SOLOSEC® (secnidazole) oral granules - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Bacterial Vaginosis - SOLOSEC is indicated for the treatment of bacterial vaginosis in female patients 12 years of age and older [see Use in Specific Populations (8.1) and Clinical ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Bacterial Vaginosis - The recommended dosage of SOLOSEC for the treatment of bacterial vaginosis in female patients 12 years of age and older is a single 2-gram ...
-
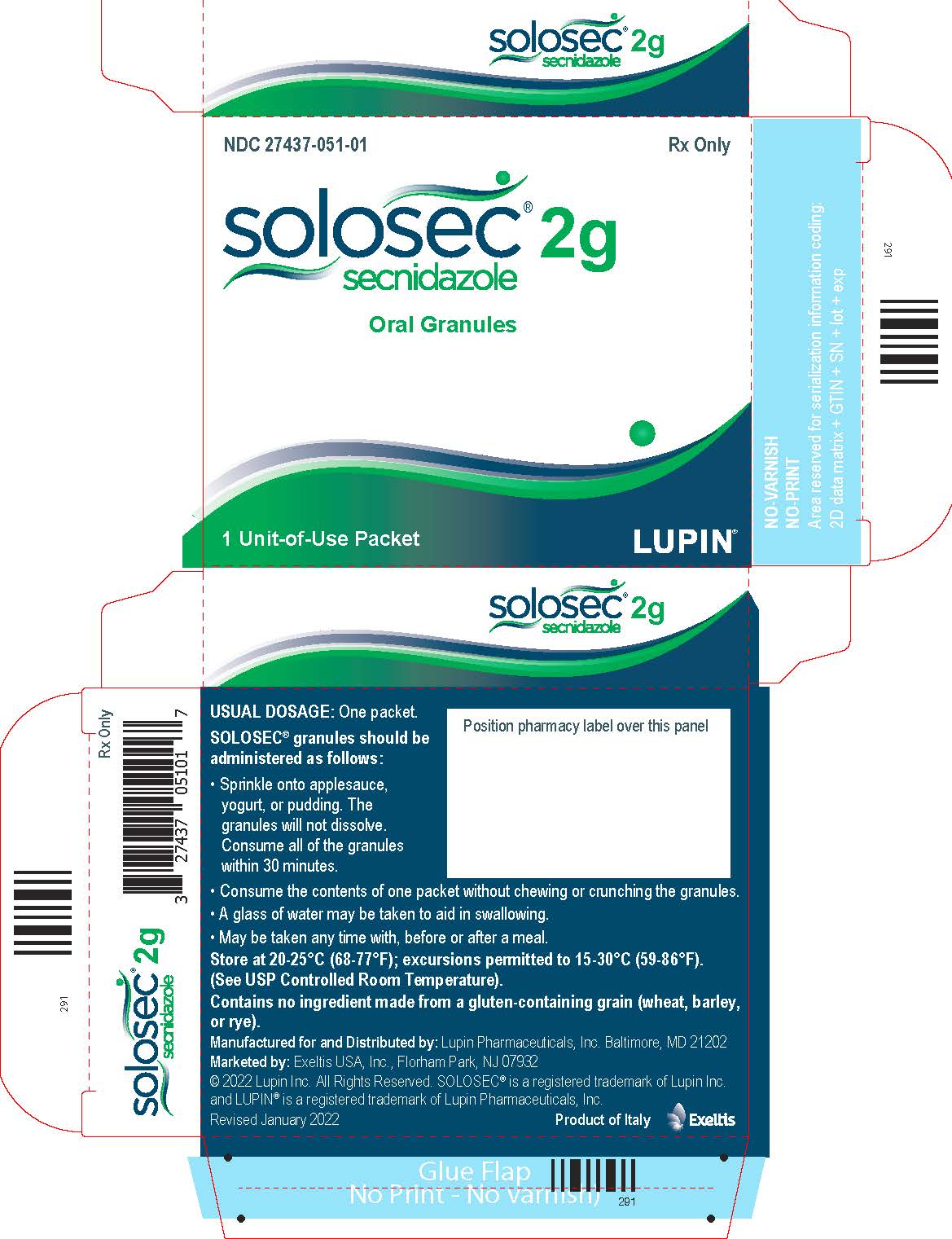
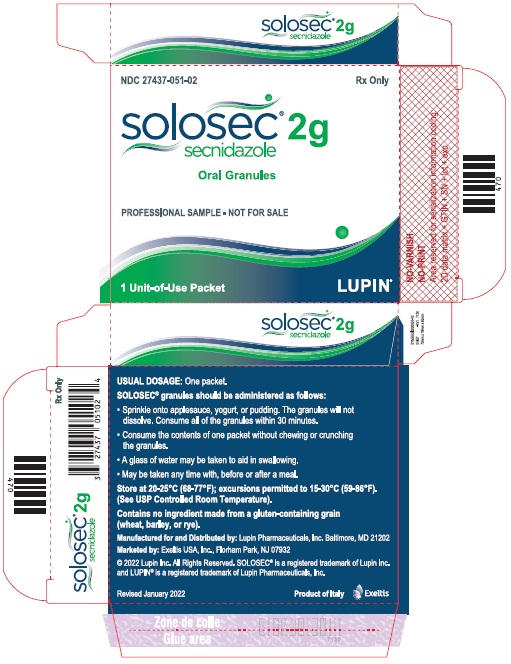
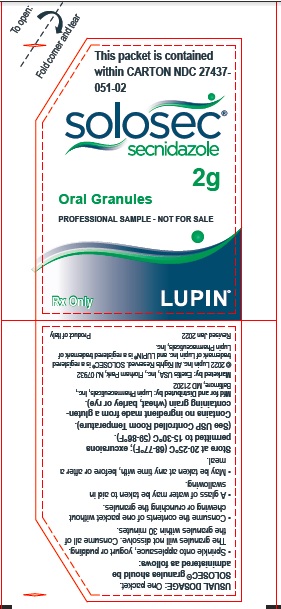
3 DOSAGE FORMS AND STRENGTHSOral Granules: 2 g, of off-white to slightly yellowish granules with 4.8 g net weight, packed in a unit-of-use child-resistant foil packet.
-
4 CONTRAINDICATIONSSOLOSEC is contraindicated: In patients who have shown hypersensitivity to secnidazole, or other nitroimidazole derivatives. In patients with Cockayne syndrome: Severe irreversible ...
-
5 WARNINGS AND PRECAUTIONS5.1 Vulvovaginal Candidiasis - The use of SOLOSEC may result in vulvovaginal candidiasis. In controlled clinical trials of non-pregnant women with bacterial vaginosis, vulvovaginal candidiasis ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are discussed in greater detail in other sections of labeling: Vulvovaginal Candidiasis [Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Oral Contraceptives - There was no clinically significant drug interaction between secnidazole and the combination oral contraceptive, ethinyl estradiol plus norethindrone [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with SOLOSEC use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal ...
-
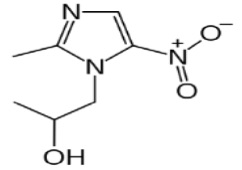
11 DESCRIPTIONThe active ingredient in SOLOSEC Oral Granules is secnidazole (also named 1-(2- hydroxypropyl)-2-methyl-5-nitroimidazole and 1-(2-methyl-5-nitro-1H-imidazol-1-yl) propan-2- ol), a nitroimidazole ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - SOLOSEC is a nitroimidazole antimicrobial drug [See Microbiology (12.4)]. 12.2 Pharmacodynamics - Secnidazole exposure-response relationships and the time course ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis Mutagenesis & Impairment of Fertility - Carcinogenesis - Nitroimidazoles, which have similar chemical structures to secnidazole, have been associated with tumors affecting the ...
-
14 CLINICAL STUDIES14.1 Bacterial Vaginosis - Two randomized placebo-controlled clinical trials (Trial 1 and Trial 2) with similar designs were conducted to evaluate the efficacy of SOLOSEC 2 gram for the ...
-
15 REFERENCES1.Özbilgin A, Özbel Y, Alkan MZ et al. Trichomoniasis in non-gonococcic urethritis among male patients. J Egypt Soc Parasitol. 1994; 24(3):621-625. 2.Dyudyun AD, Polyon NM, Gorbuntsov VV ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSOLOSEC (secnidazole) Oral Granules, 2 g, consists of off-white to slightly yellowish granules containing secnidazole. SOLOSEC is supplied in one unit-of-use child-resistant foil packet of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Administration Instructions - Instruct the patient: To sprinkle the entire contents ...
-
PATIENT INFORMATIONSOLOSEC® (SO-lo-sec) (secnidazole) oral granules - What is SOLOSEC? SOLOSEC is a prescription medicine used to treat: bacterial vaginal infections in females 12 years of age and ...
-
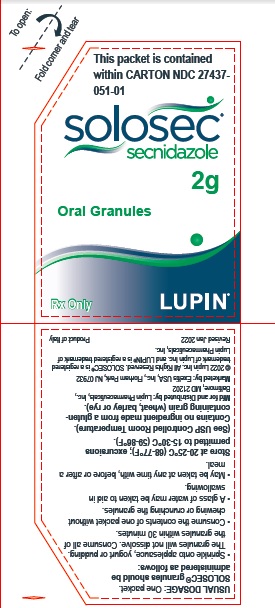
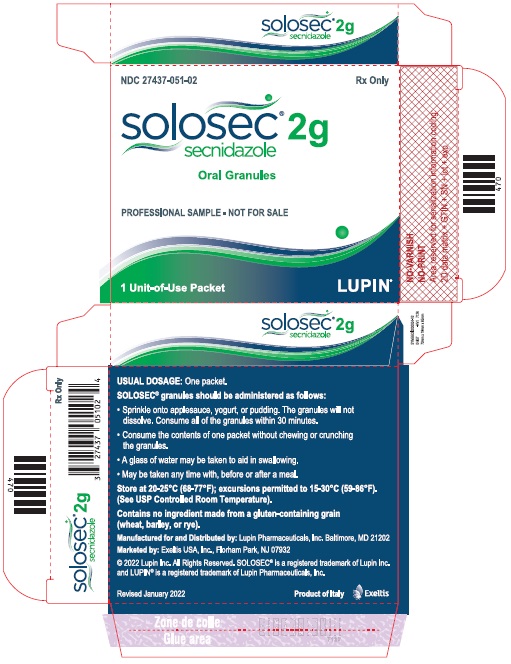
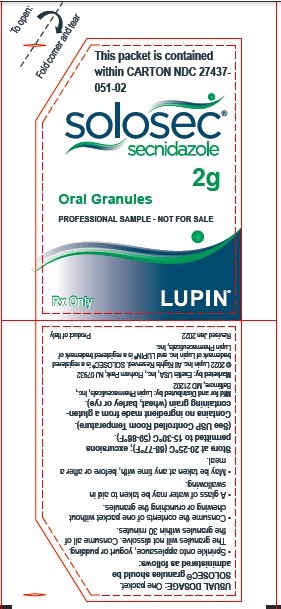
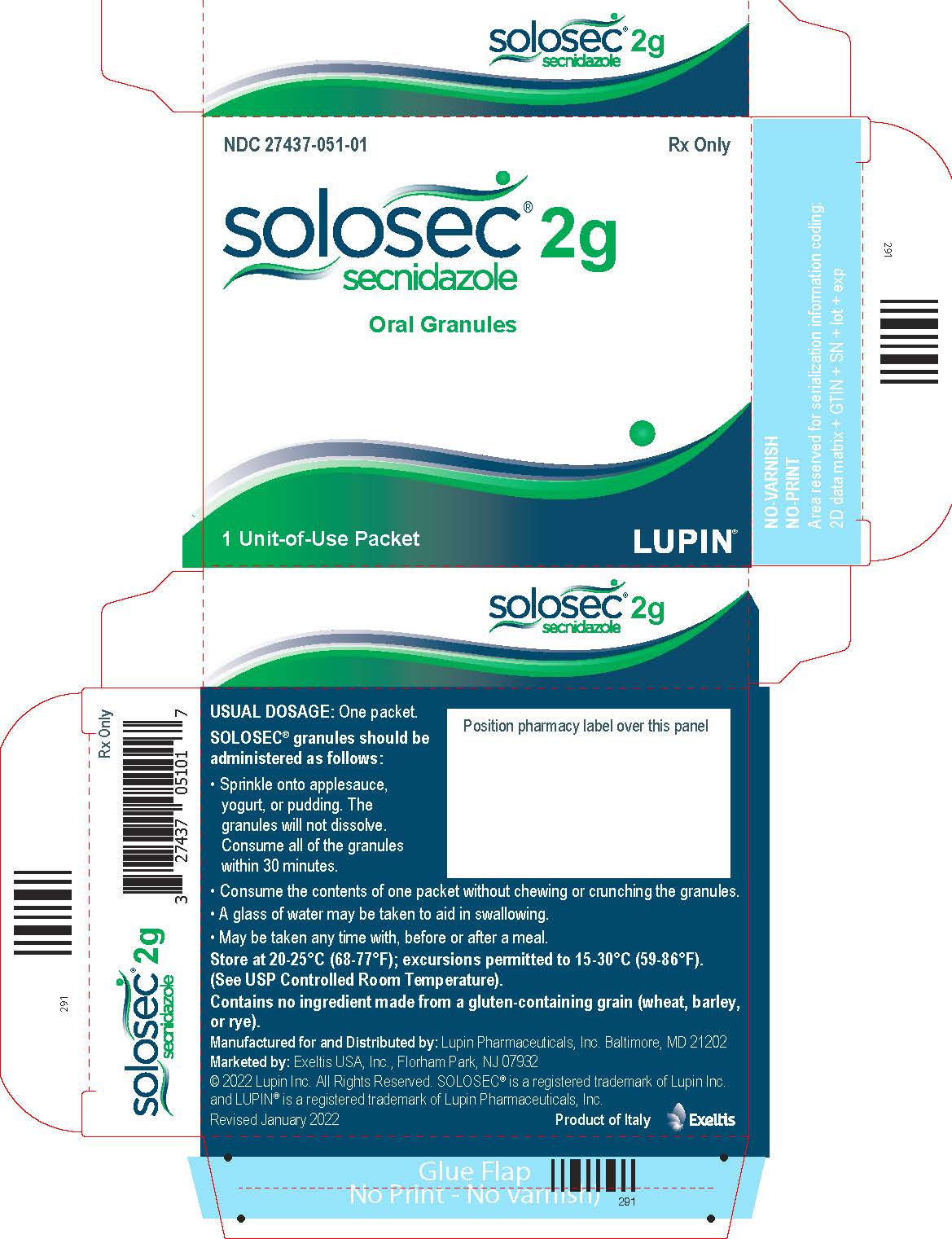
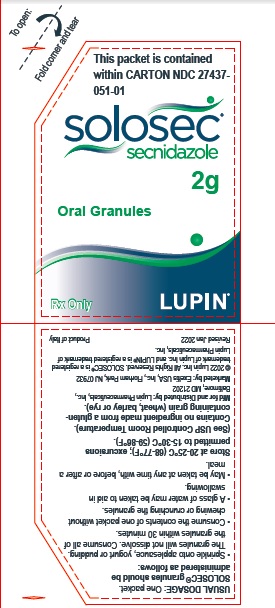
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPrincipal Display Panel - Individual Carton - SOLOSEC® 2g - secnidazole - NDC 27437-051-01 - Oral Granules - 1 Unit-of-Use Packet - Rx Only - LUPIN PHARMACEUTICALS, INC. USUAL DOSAGE: One packet ...
-
INGREDIENTS AND APPEARANCEProduct Information