Label: SOLODYN- minocycline hydrochloride tablet, film coated, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-6285-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 99207-466
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 4, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOLODYN safely and effectively. See full prescribing information for SOLODYN. SOLODYN® (minocycline HCl) Extended Release ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - SOLODYN is indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older. 1.2 Limitations of Use - SOLODYN ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of SOLODYN is approximately 1 mg/kg once daily for 12 weeks. Higher doses have not shown to be of additional benefit in the treatment of inflammatory lesions of acne, and ...
-
3 DOSAGE FORMS AND STRENGTHS45 mg extended release tablets: gray, unscored, coated, and debossed with "DYN-045" on one side. 55 mg extended release tablets: pink, unscored, coated, and debossed with "DYN-055" on one ...
-
4 CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
5 WARNINGS AND PRECAUTIONS5.1 Teratogenic Effects - MINOCYCLINE, LIKE OTHER TETRACYCLINE-CLASS DRUGS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY OR IF THE ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under prescribed conditions, adverse reaction rates observed in the clinical trial may not reflect the rates observed in ...
-
7 DRUG INTERACTIONS7.1 Anticoagulants - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects: Pregnancy category D [see Warnings and Precautions (5.1)] SOLODYN should not be used during pregnancy. If the patient becomes pregnant while taking this ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Minocycline is not removed in significant quantities by hemodialysis or peritoneal ...
-
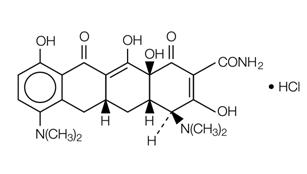
11 DESCRIPTIONMinocycline hydrochloride, a semi synthetic derivative of tetracycline, is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of SOLODYN for the treatment of acne is unknown. 12.2 Pharmacodynamics - The pharmacodynamics of SOLODYN for the treatment of acne are ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis—Long-term animal studies have not been performed to evaluate the carcinogenic potential of minocycline. A ...
-
14 CLINICAL STUDIESThe safety and efficacy of SOLODYN in the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris was assessed in two 12-week, multi-center, randomized, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - SOLODYN (minocycline HCl, USP) Extended Release Tablets are supplied as aqueous film coated tablets containing minocycline hydrochloride equivalent to 80 mg minocycline, are ...
-
17 PATIENT COUNSELING INFORMATION[See FDA-approved patient labeling (Patient Information)] Patients taking SOLODYN (minocycline HCl, USP) Extended Release Tablets should receive the following information and instructions ...
-
PATIENT PACKAGE INSERTPatient Information - SOLODYN® (SO-lo-dīn) (minocycline HCl) Extended Release Tablets - Read this Patient Information leaflet that comes with SOLODYN before you start taking it and each time you get ...
-
SPL UNCLASSIFIED SECTIONRevised: 03/2011 - U.S. Patent 5,908,838, U.S. Patent 7,790,705 and Patents Pending1 - © 2010 Medicis Pharmaceutical Corporation - SOLODYN is a registered trademark of Medicis Pharmaceutical ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 80 mg Bottle Label - Rx Only - SOLODYN® (MINOCYCLINE HCl, USP) EXTENDED RELEASE TABLETS - 80 mg*
-
INGREDIENTS AND APPEARANCEProduct Information