Label: SINUVA- mometasone furoate implant
- NDC Code(s): 10599-003-01
- Packager: Intersect ENT, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SINUVA® safely and effectively. See full prescribing information for SINUVA. SINUVA (mometasone furoate) sinus implant - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESINUVA Sinus Implant is indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients ≥18 years of age who have had ethmoid sinus surgery.
-
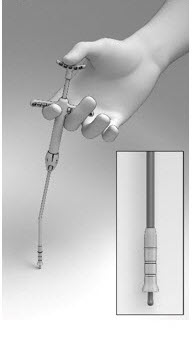
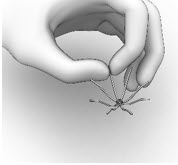
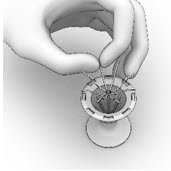
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage is one SINUVA Sinus Implant (1350 mcg of mometasone furoate) placed in an ethmoid sinus [see Dosage and Administration (2.3)]. The SINUVA Sinus ...
-
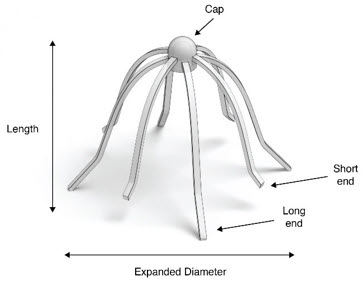
3 DOSAGE FORM AND STRENGTHSImplant: a sterile, single-use, bioabsorbable implant, coated with a formulation containing 1350 mcg mometasone furoate that is gradually released over 90 days.
-
4 CONTRAINDICATIONSPatients with known hypersensitivity to mometasone furoate, or to any of the copolymers of the SINUVA Sinus Implant [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Local Nasal Adverse Reactions - Monitor nasal mucosa adjacent to the SINUVA Sinus Implant for any signs of bleeding (epistaxis), irritation, infection, or perforation. Avoid use in patients ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Local Nasal Adverse Reactions [see Warnings and Precautions (5.1)] Glaucoma and Cataracts [see ...
-
7 DRUG INTERACTIONSFormal drug-drug interaction studies have not been conducted with the SINUVA Sinus Implant. An evaluation of the concurrent administration of the SINUVA Sinus Implant and other commonly used nasal ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no randomized clinical studies of SINUVA Sinus Implant or mometasone furoate in pregnant women. The active pharmaceutical ingredient, mometasone ...
-
10 OVERDOSAGEThere are no data available on the effects of acute or chronic overdosage with SINUVA Sinus Implant. Chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions ...
-
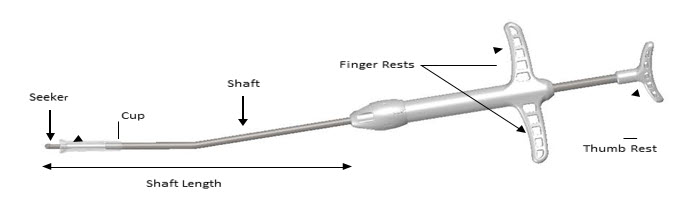
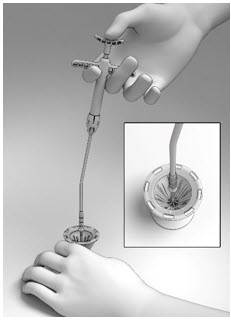
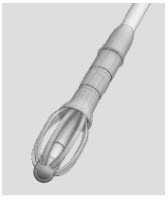
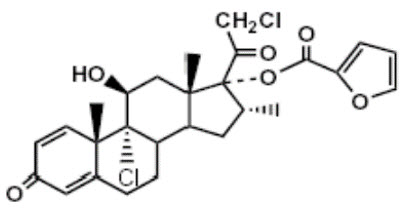
11 DESCRIPTIONThe SINUVA Sinus Implant is a self-expanding, bioabsorbable, drug eluting implant provided with a crimper and a single-use delivery system. SINUVA Sinus Implant is comprised of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on inflammation is not known ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase of ...
-
14 CLINICAL STUDIESThe SINUVA Sinus Implant was evaluated in 450 patients, 18 years of age and older, with chronic rhinosinusitis with nasal polyps and a history of ethmoid sinus surgery. The development program ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGA carton of SINUVA contains one SINUVA (1350 mcg mometasone furoate) implant inside of a Crimper and one disposable Delivery System packaged in a foil pouch. The SINUVA Sinus Implant is 20 mm in ...
-
17 PATIENT COUNSELING INFORMATIONEncourage patients to use saline irrigations or sprays regularly. Advise the patient that the Implant is bioabsorbable and intended to soften over time. As the Implant softens and polyps ...
-
SPL UNCLASSIFIED SECTIONThis product and/or the use of this product in a method may be covered by one or more patents or patent applications, available at www.sinuva.com - © 2020 Intersect ENT, Inc. All rights reserved ...
-
PRINCIPAL DISPLAY PANEL - 1350 mcg Implant Pouch CartonSINUVA™ (mometasone furoate) sinus implant
-
INGREDIENTS AND APPEARANCEProduct Information