Label: VEREGEN- sinecatechins ointment
- NDC Code(s): 62559-385-02, 62559-385-26, 62559-385-30
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VEREGEN safely and effectively. See full prescribing information for VEREGEN. Veregen® (sinecatechins) ointment, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - Veregen is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older. 1.2 Limitations ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Veregen is to be applied three times per day to all external genital and perianal warts. Apply about an 0.5 cm strand of the Veregen to each wart using the ...
-
3 DOSAGE FORMS AND STRENGTHSOintment, 15% w/w. Each gram of Veregen Ointment, 15% contains 150 mg of sinecatechins in a brown ointment base.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONSVeregen has not been evaluated for the treatment of urethral, intra-vaginal, cervical, rectal, or intra-anal human papilloma viral disease and should not be used for the treatment of these ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Veregen use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
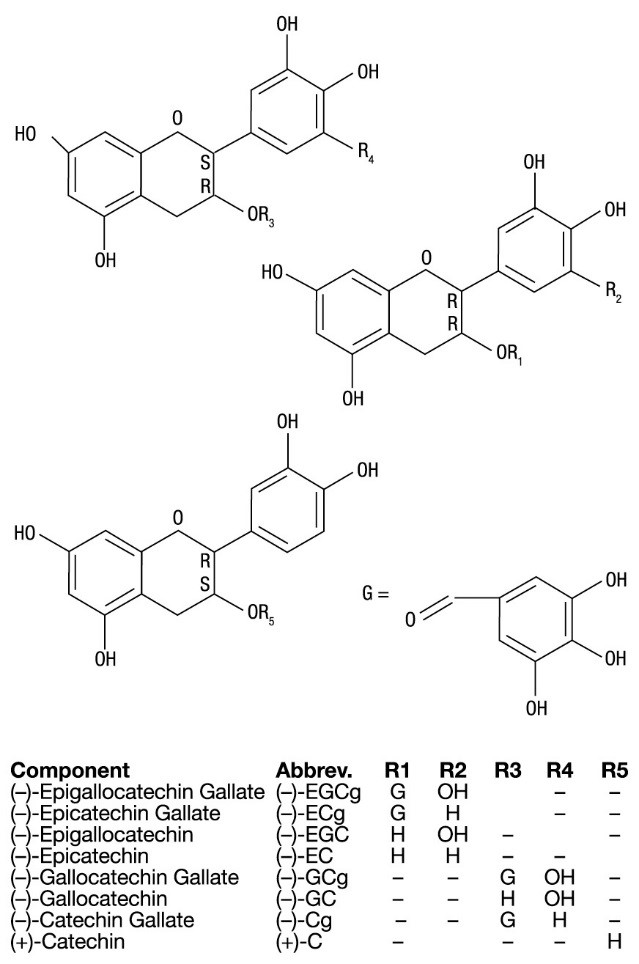
11 DESCRIPTIONVeregen (sinecatechins) Ointment, 15% is a botanical drug product for topical use. The drug substance in Veregen is sinecatechins, which is a partially purified fraction of the water extract of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mode of action of Veregen involved in the clearance of genital and perianal warts is unknown. In vitro, sinecatechins had anti-oxidative activity; the clinical ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In an oral (gavage) carcinogenicity study, sinecatechins was administered daily for 26 weeks to p53 transgenic mice at doses up to 500 ...
-
14 CLINICAL STUDIESTwo randomized, double-blind, vehicle-controlled trials were performed to investigate the safety and efficacy of Veregen in the treatment of immunocompetent subjects 18 years of age and older with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVeregen is a brown ointment and is supplied in an aluminum tube containing 30 grams (NDC 62559-385-30) of ointment per tube. Prior to dispensing to the patient, store refrigerated 2°C to 8°C ...
-
17 PATIENT COUNSELING INFORMATION See FDA-approved patient labeling (Patient Information) Patients using Veregen should receive the following information and instructions: • This medication is only to be used as directed by a ...
-
Patient Package Insert PATIENT INFORMATION - Veregen® (sinecatechins) Ointment, 15% Read this leaflet carefully before you start using Veregen Ointment and each time you refill your prescription. There may be new ...
-
PRINCIPAL DISPLAY PANELVEREGEN® (sinecatechins) Ointment - 15 % For Topical Use Only - 30g
-
INGREDIENTS AND APPEARANCEProduct Information