Label: SIMULECT- basiliximab injection, powder, for solution
- NDC Code(s): 0078-0331-84, 0078-0393-61
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Only physicians experienced in immunosuppression therapy and management of organ transplantation patients should prescribe Simulect® (basiliximab). The physician responsible for Simulect administration should have complete information requisite for the follow-up of the patient. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources.
Close -
DESCRIPTION
Basiliximab is a chimeric (murine/human) monoclonal antibody (IgG1к), produced by recombinant DNA technology, that functions as an immunosuppressive agent, specifically binding to and blocking the ...
-
CLINICAL PHARMACOLOGY
General - Mechanism of Action: Basiliximab functions as an IL-2 receptor antagonist by binding with high affinity (Ka = 1 x 1010 M-1) to the alpha chain of the high affinity IL-2 receptor ...
-
CLINICAL STUDIES
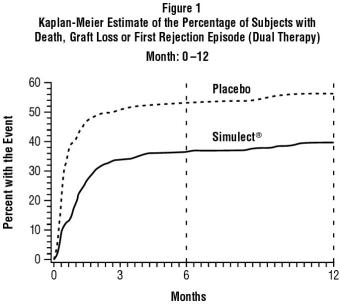
The safety and efficacy of Simulect® (basiliximab) for the prophylaxis of acute organ rejection in adults following cadaveric- or living-donor renal transplantation were assessed in four ...
-
INDICATIONS AND USAGE
Simulect® (basiliximab) is indicated for the prophylaxis of acute organ rejection in patients receiving renal transplantation when used as part of an immunosuppressive regimen that includes ...
-
CONTRAINDICATIONS
Simulect® (basiliximab) is contraindicated in patients with known hypersensitivity to basiliximab or any other component of the formulation. See composition of Simulect under ...
-
PRECAUTIONS
General - It is not known whether Simulect® (basiliximab) use will have a long-term effect on the ability of the immune system to respond to antigens first encountered during Simulect-induced ...
-
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
OVERDOSAGE
A maximum tolerated dose of Simulect® (basiliximab) has not been determined in patients. During the course of clinical studies, Simulect has been administered to adult renal transplantation ...
-
DOSAGE AND ADMINISTRATION
Simulect® (basiliximab) is used as part of an immunosuppressive regimen that includes cyclosporine, USP (MODIFIED) and corticosteroids. Simulect is for central or peripheral intravenous ...
-
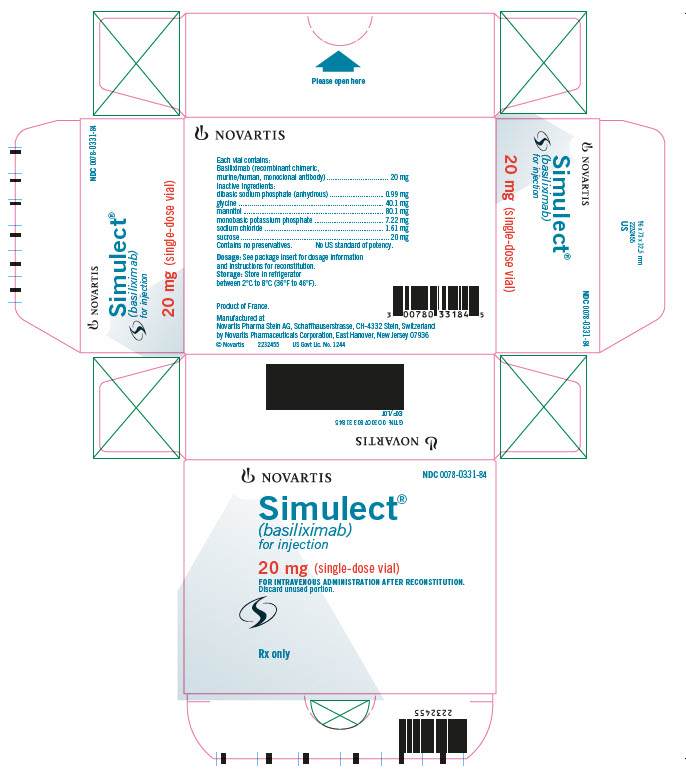
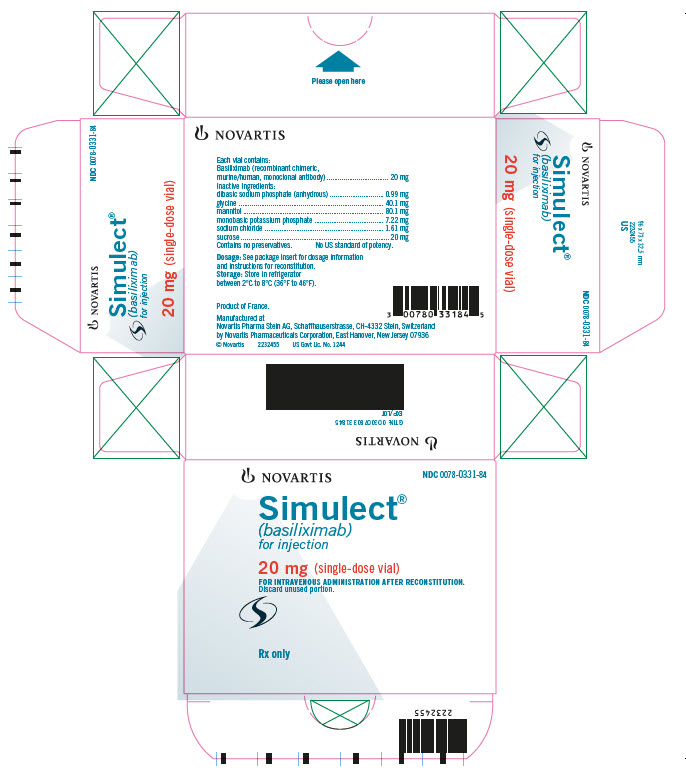
HOW SUPPLIED
Simulect® (basiliximab) is supplied in a single-dose glass vial. Each carton contains one of the following - 1 Simulect 10 mg vial…………………………………………………….NDC 0078-0393-61 - 1 Simulect 20 mg ...
-
REFERENCES
Kahan, B.D., Rajagopalan P.R. and Hall M., Transplantation, 67, 276-284 (1999). Nashan, B., Moore R., Amlot P., Schmidt A.-G., Abeywickrama K. and Soulillou J.-P., Lancet 350, 1193-1198 ...
-
PRINCIPAL DISPLAY PANELNOVARTIS NDC 0078-0393-61 - Simulect® (basiliximab) for injection - 10 mg (single-dose vial) FOR INTRAVENOUS ADMINISTRATION AFTER RECONSTITUTION. Discard unused portion. Rx only
-
PRINCIPAL DISPLAY PANELNOVARTIS NDC 0078-0331-84 - Simulect® (basiliximab) for injection - 20 mg (single-dose vial) FOR INTRAVENOUS ADMINISTRATION AFTER RECONSTITUTION. Discard unused portion. Rx only
-
INGREDIENTS AND APPEARANCEProduct Information