Label: SIMBRINZA- brinzolamide/brimonidine tartrate suspension/ drops
- NDC Code(s): 0065-4147-25, 0065-4147-27
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SIMBRINZA® safely and effectively. See full prescribing information for SIMBRINZA. SIMBRINZA® (brinzolamide and brimonidine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is a fixed combination of a carbonic anhydrase inhibitor and an alpha 2 adrenergic receptor agonist indicated for the ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose is one drop of SIMBRINZA in the affected eye(s) three times daily. Shake well before use. SIMBRINZA ophthalmic suspension may be used concomitantly with other topical ...
-
3 DOSAGE FORMS & STRENGTHSOphthalmic suspension containing 1% (10 mg/mL) brinzolamide and 0.2% (2 mg/mL) brimonidine tartrate.
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - SIMBRINZA is contraindicated in patients who are hypersensitive to any component of this product. 4.2 Neonates and Infants (under the age of two years) SIMBRINZA is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Sulfonamide Hypersensitivity Reactions - SIMBRINZA contains brinzolamide, a sulfonamide, and although administered topically is absorbed systemically. Therefore, the same types of adverse ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Oral Carbonic Anhydrase Inhibitors - There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Developmental toxicity studies with brinzolamide in rabbits at oral doses of 1, 3, and 6 mg/kg/day (20, 60, and 120 times the recommended human ophthalmic dose) produced maternal ...
-
10 OVERDOSAGEAlthough no human data are available, electrolyte imbalance, development of an acidotic state, and possible nervous system effects may occur following an oral overdose of brinzolamide. Serum ...
-
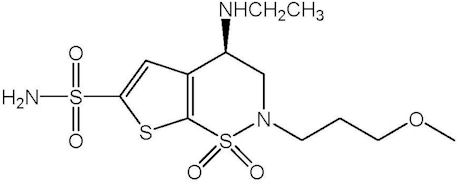
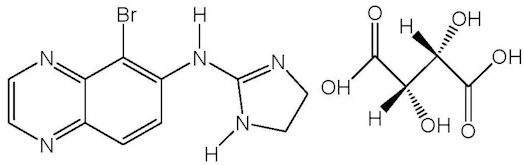
11 DESCRIPTIONSIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is a fixed combination of a carbonic anhydrase inhibitor and an alpha 2 adrenergic receptor agonist for topical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - SIMBRINZA is comprised of two components: brinzolamide (carbonic anhydrase inhibitor) and brimonidine tartrate (alpha 2 adrenergic receptor agonist). Each of these two ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Brinzolamide caused urinary bladder tumors in female mice at oral doses of 10 mg/kg/day and in male rats at oral doses of 8 mg/kg/day ...
-
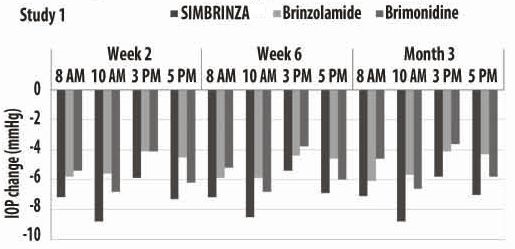
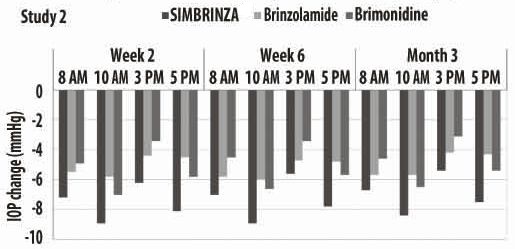
14 CLINICAL STUDIESTwo clinical trials of 3 months duration were conducted in patients with open-angle glaucoma or ocular hypertension to compare the IOP-lowering effect of SIMBRINZA (brinzolamide/brimonidine ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is supplied in white low density polyethylene (LDPE) DROP-TAINER® bottles with a natural LDPE dispensing-tip and light ...
-
17 PATIENT COUNSELING INFORMATIONSulfonamide Hypersensitivity Reactions - Advise patients that if serious or unusual ocular or systemic reactions or signs of hypersensitivity occur, they should discontinue the use of the ...
-
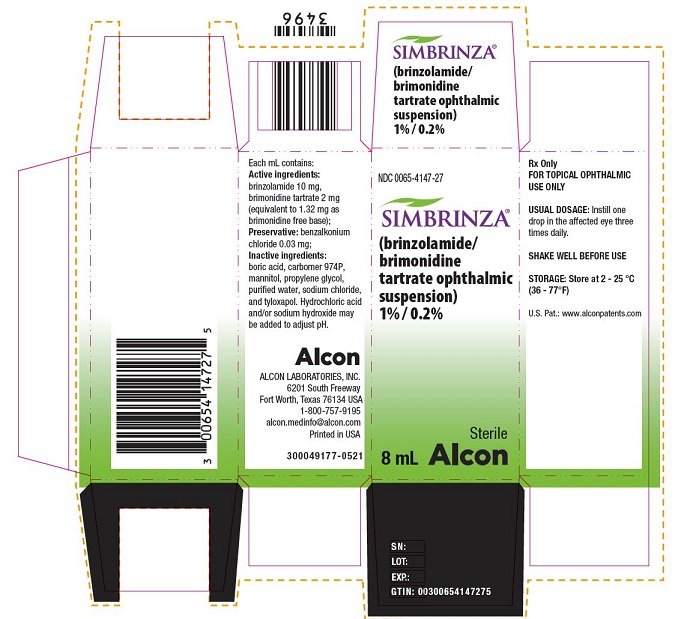
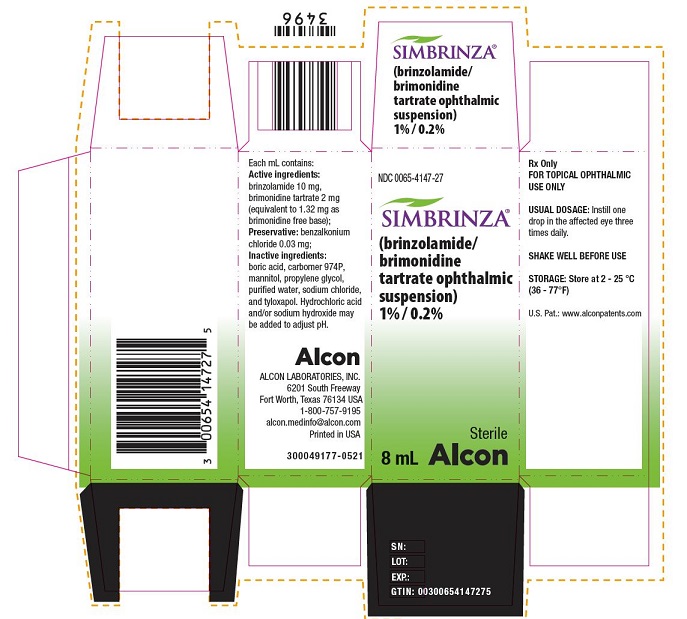
PRINCIPAL DISPLAY PANELNDC 0065-4147-27 - SIMBRINZA® (brinzolamide/brimonidine tartrate ophthalmic suspension) 1% / 0.2% Sterile - 8 mL - Alcon - Rx Only - FOR TOPICAL OPHTHALMIC USE ONLY - USUAL ...
-
INGREDIENTS AND APPEARANCEProduct Information