Label: SILIQ- brodalumab injection
- NDC Code(s): 0187-0004-00, 0187-0004-02
- Packager: Bausch Health US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SILIQ safely and effectively. See full prescribing information for SILIQ. SILIQ - ®(brodalumab) injection, for subcutaneous use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL IDEATION AND BEHAVIOR
Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with SILIQ. Prior to prescribing SILIQ, weigh the potential risks and benefits in patients with a history of depression and/or suicidal ideation or behavior. Patients with new or worsening suicidal ideation and behavior should be referred to a mental health professional, as appropriate. Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes [ see Warnings and Precautions( 5.1) ].
Because of the observed suicidal behavior in subjects treated with SILIQ, SILIQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the SILIQ REMS Program [see Warnings and Precautions( 5.2) ].
Close -
1 INDICATIONS AND USAGESILIQ - ®(brodalumab) is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond ...
-
2 DOSAGE AND ADMINISTRATION2.1 Tuberculosis Assessment Prior to Initiation of SILIQ - Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with SILIQ - [see Warnings and Precautions ...
-
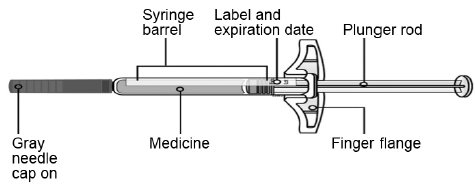
3 DOSAGE FORMS AND STRENGTHSInjection: 210 mg/1.5 mL solution in a single-dose prefilled syringe. SILIQ is a clear to slightly opalescent, colorless to slightly yellow solution.
-
4 CONTRAINDICATIONSSILIQ is contraindicated in patients with: Crohn’s disease because SILIQ may cause worsening of disease [see Warnings and Precautions ( 5.7)]. Clinically significant ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Ideation and Behavior - Suicidal ideation and behavior, including four completed suicides, occurred in subjects treated with SILIQ in the psoriasis clinical trials. There were no ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of labeling: Suicidal Ideation and Behavior - [see Warnings and Precautions ( 5.1) ...
-
7 DRUG INTERACTIONSCYP450 Substrates - The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation. Treatment with SILIQ ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no human data on SILIQ use in pregnant women to inform a drug-associated risk. Human IgG antibodies are known to cross the placental barrier; therefore ...
-
11 DESCRIPTIONBrodalumab is a human monoclonal IgG2κ antibody directed against human interleukin-17 receptor A (IL-17RA). It is expressed in a Chinese Hamster Ovary (CHO) cell line. Brodalumab is comprised of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Brodalumab is a human monoclonal IgG2 antibody that selectively binds to human IL-17RA and inhibits its interactions with cytokines IL-17A, IL-17F, IL-17C, IL-17A/F ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of SILIQ. The published literature is mixed ...
-
14 CLINICAL STUDIESThree multicenter, randomized, double-blind, controlled trials (Trials 1, 2, and 3) enrolled a total of 4373 subjects 18 years of age and older with at least a 6-month history of moderate to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - SILIQ - ®(brodalumab) injection is available in a single-dose prefilled syringe containing a sterile, preservative-free clear to slightly opalescent, colorless to slightly yellow ...
-
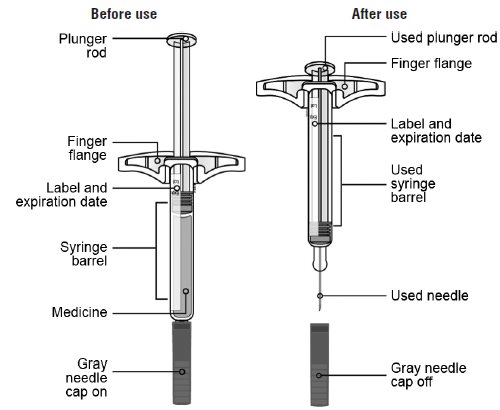
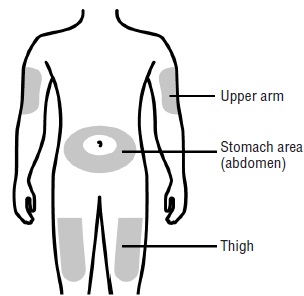
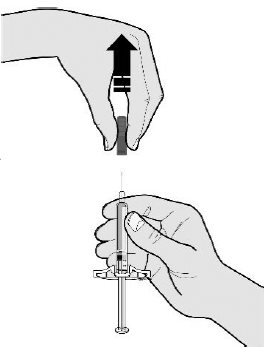
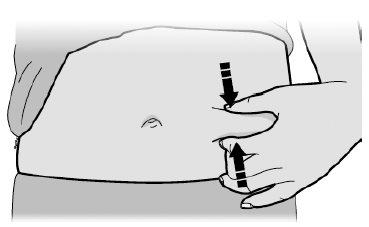
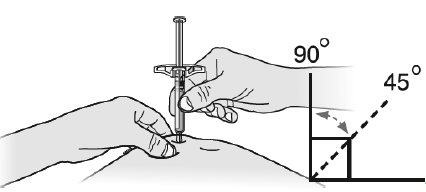
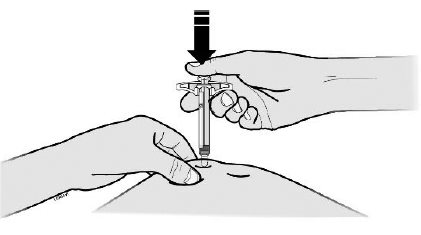
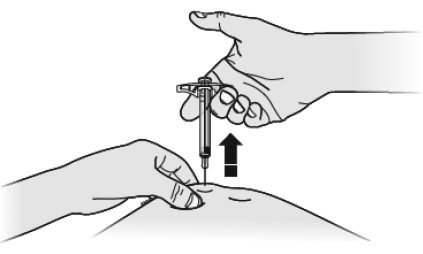
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling ( Medication Guideand - Instructions for Use) before the patient starts using SILIQ, and each time the prescription is renewed ...
-
Medication GuideMEDICATION GUIDE - Siliq - ®(sil-eek) (brodalumab) Injection, for subcutaneous use - What is the most important information I should know about ...
-
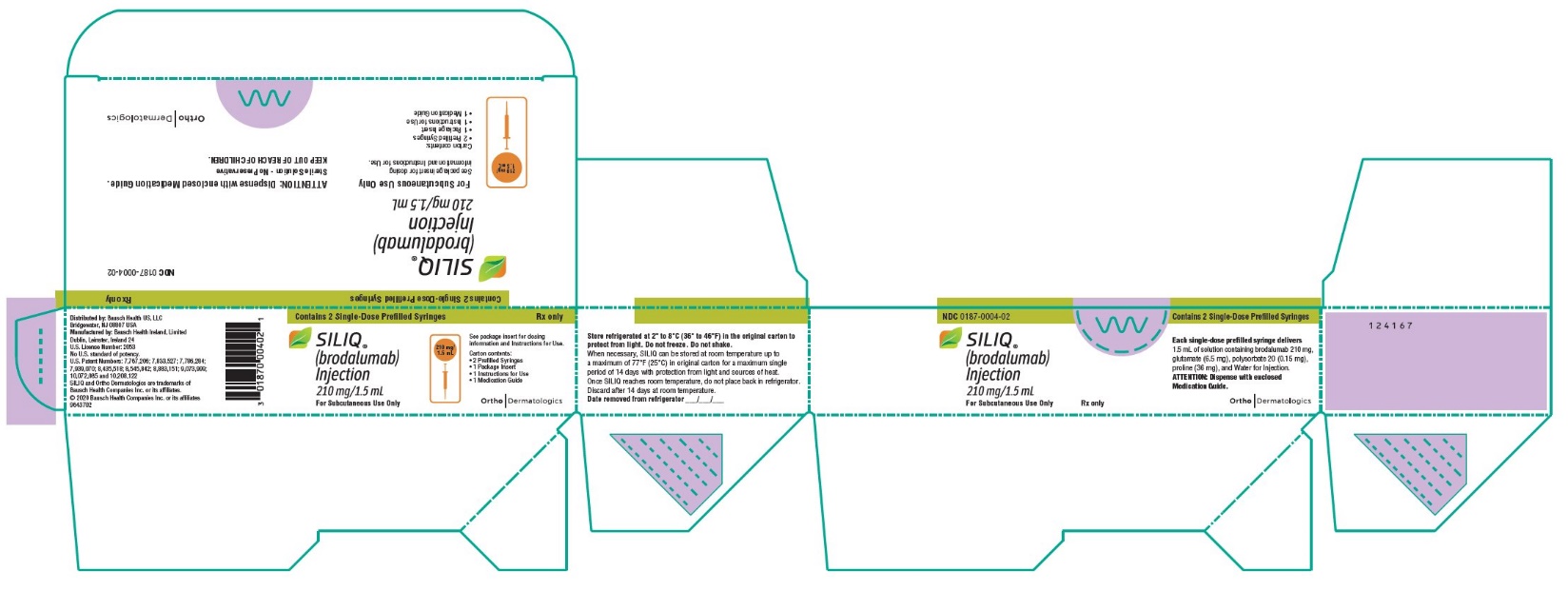
PACKAGE/LABEL PRINCIPAL DISPLAY PANELContains 2 Single-Dose Prefilled Syringes - NDC0187-0004-02 - SILIQ - ® (brodalumab) Injection - 210 mg/1.5 mL - For Subcutaneous Use Only - See package insert for ...
-
INGREDIENTS AND APPEARANCEProduct Information