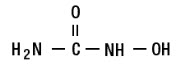Label: SIKLOS- hydroxyurea tablet, film coated
- NDC Code(s): 71770-105-60, 71770-120-30
- Packager: Medunik
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SIKLOS safely and effectively. See full prescribing information for SIKLOS. SIKLOS (hydroxyurea) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Myelosuppression:SIKLOS may cause severe myelosuppression. Monitor blood counts at baseline and throughout treatment. Interrupt treatment and reduce dose as necessary - [see - Warnings ...
WARNING: MYELOSUPPRESSION and MALIGNANCIES
Myelosuppression:SIKLOS may cause severe myelosuppression. Monitor blood counts at baseline and throughout treatment. Interrupt treatment and reduce dose as necessary [see Warnings and Precautions (5.1)] .
CloseMalignancies:Hydroxyurea is carcinogenic. Advise sun protection and monitor patients for malignancies [see Warnings and Precautions (5.2)] .
-
1 INDICATIONS AND USAGESIKLOS - ®is indicated to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult and pediatric patients, 2 years of age and older, with sickle cell ...
-
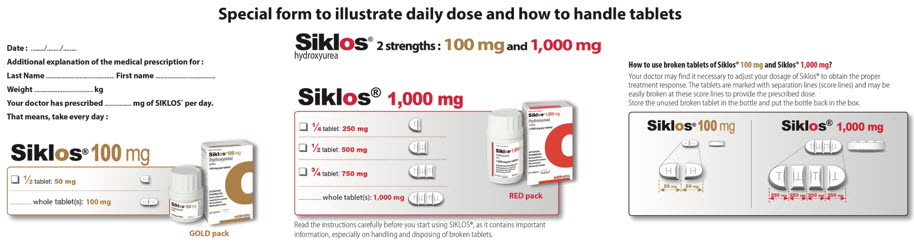
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - The recommended SIKLOS dosing is described in Table 1. Table 1: Dosing Recommendation Based on Blood Count - Dosing RegimenDoseDose Modification CriteriaMonitoring ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 100 mg tablets: off-white, capsule-shaped, film-coated, functionally scored tablet with scoring on both sides which can be divided into two equal parts, each part is debossed with "H ...
-
4 CONTRAINDICATIONSSIKLOS is contraindicated in: Patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation - [see - Adverse Reactions (6)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Hydroxyurea causes severe myelosuppression. Do not initiate treatment with hydroxyurea in patients if bone marrow function is markedly depressed. Bone marrow suppression ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression - [see - Warnings and Precautions (5.1)] Malignancies ...
-
7 DRUG INTERACTIONS7.1 Increased Toxicity with Concomitant Use of Antiretroviral Drugs - Pancreatitis - Pancreatitis (including fatal cases) have occurred in patients with HIV infection during therapy with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - SIKLOS can cause fetal harm based on findings from animal studies and the drug's mechanism of action - [see - Clinical Pharmacology (12.1)] . There are ...
-
10 OVERDOSAGEAcute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at doses several times above the therapeutic dose. Soreness, violet erythema, oedema on palms and soles followed by ...
-
11 DESCRIPTIONSIKLOS (hydroxyurea) is an antimetabolite that is available for oral use as functionally scored 100 mg film-coated tablet and functionally triple-scored 1,000 mg film-coated tablet containing 100 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which hydroxyurea produces its cytotoxic and cytoreductive effects is not known. However, various studies support the hypothesis that ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Conventional long-term studies to evaluate the carcinogenic potential of hydroxyurea have not been performed. However, hydroxyurea is ...
-
14 CLINICAL STUDIESPediatric Patients with Sickle Cell Disease - The efficacy of SIKLOS was assessed in the European Sickle Cell Disease Cohort study (ESCORT HU) [NCT02516579]. This is an open-label single-arm ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA.http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - SIKLOS (hydroxyurea) film-coated tablet is supplied in high density polyethylene (HDPE) bottle with polypropylene child-resistant cap with a desiccant unit containing 30 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Instructions for Use and Medication Guide). There is a risk of myelosuppression. Emphasize the importance of monitoring ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Medunik USA, 2 Research Way, suite 1B, Princeton, NJ 08540. Manufactured for. Theravia: 16 rue Montrosier 92200 Neuilly-sur-Seine France - Manufactured by: Delpharm ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 11/2023 - MEDICATION GUIDE - SIKLOS (See – k – los) (hydroxyurea) tablets ...
-
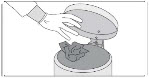
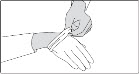
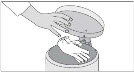
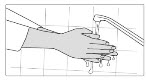
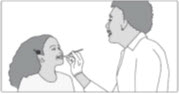
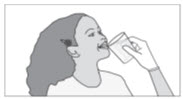
INSTRUCTIONS FOR USESIKLOS (See – k – los) (hydroxyurea) tablets - Read this Instructions for Use before you start taking SIKLOS and each time you get a refill. There may be new information. This ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg Tablet Bottle LabelNDC 71770-120-30 - Siklos - ®1,000 mg - (hydroxyurea) tablets - 1,000 mg per tablet - ATTENTION PHARMACIST: Each patient is required - to receive the enclosed ...
-
PRINCIPAL DISPLAY PANEL - 1,000 mg Tablet Bottle CartonNDC 71770-120-30 - Siklos - ®1,000 mg - (hydroxyurea) tablets - 1,000 mg per tablet - ATTENTION - PHARMACIST: Each patient is - required to receive - the ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelSiklos - ® 100 mg - (hydroxyurea) 100 mg per tablet - 60 tablets - NDC 71770-105-60 - Store at 20°C to 25°C (68°F to 77°F) Wear disposable gloves when handling ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle CartonNDC 71770-105-60 - Siklos - ®100 mg - (hydroxyurea) tablets - 100 mg per tablet - ATTENTION - PHARMACIST: Each patient is - required to receive - the ...
-
INGREDIENTS AND APPEARANCEProduct Information