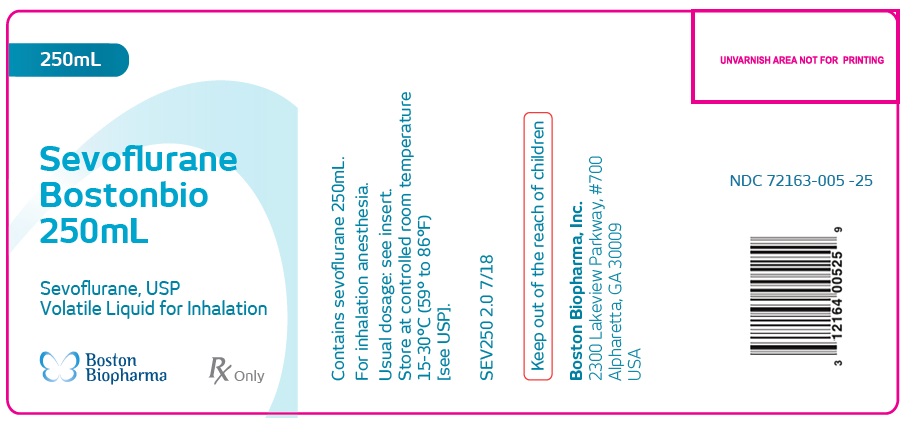
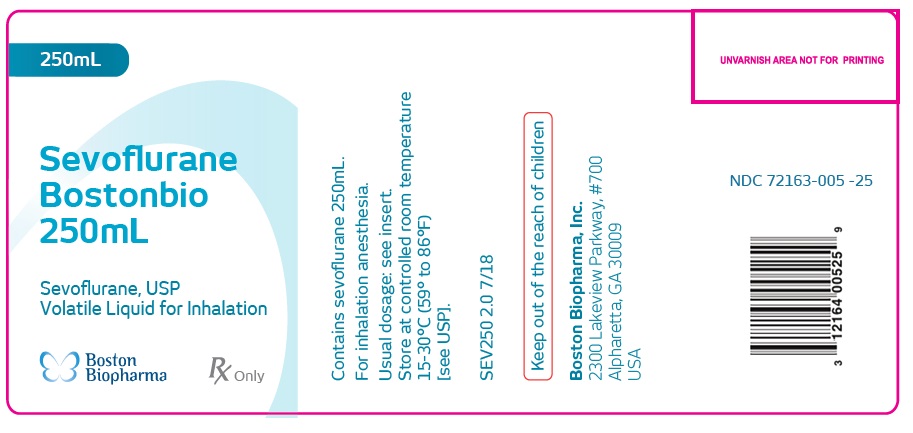
Label: SEVOFLURANE BOSTONBIO- sevoflurane inhalant
-
Contains inactivated NDC Code(s)
NDC Code(s): 72163-005-25 - Packager: Boston Biopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HOW SUPPLIEDSevoflurane - Bostonbio - 250mL - Sevoflurane, USP - Volatile Liquid for Inhalation - Contains sevoflurane 250mL ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELSevoflurane - Bostonbio - 250mL - Sevoflurane, USP - Volatile Liquid for Inhalation
-
INGREDIENTS AND APPEARANCEProduct Information