Label: SEROSTIM- somatropin kit
- NDC Code(s): 44087-0004-7, 44087-0005-7, 44087-0006-7
- Packager: EMD Serono, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SEROSTIM® safely and effectively. See full prescribing information for SEROSTIM. SEROSTIM (somatropin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESEROSTIM (somatropin) is indicated for the treatment of HIV patients with wasting or cachexia to increase lean body mass and body weight, and improve physical endurance. Concomitant antiretroviral ...
-
2 DOSAGE AND ADMINISTRATIONSEROSTIM is administered by subcutaneous injection. SEROSTIM therapy should be carried out under the regular guidance of a physician who is experienced in the diagnosis and management of HIV ...
-
3 DOSAGE FORMS AND STRENGTHSSingle-use administration (to be reconstituted with Sterile Water for Injection): SEROSTIM 5 mg per vial - SEROSTIM 6 mg per vial - Multi-use administration (to be reconstituted with Bacteriostatic ...
-
4 CONTRAINDICATIONSAcute Critical Illness - Growth hormone therapy should not be initiated in patients with acute critical illness due to complications following open heart or abdominal surgery, multiple accidental ...
-
5 WARNINGS AND PRECAUTIONS5.1 Acute Critical Illness - Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are also described elsewhere in the labeling: Acute Critical Illness [see Warnings and Precautions (5.1)] Neoplasms [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSFormal drug interaction studies have not been conducted. No data are available on drug interactions between SEROSTIM and HIV protease inhibitors or the non-nucleoside reverse transcriptase ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Reproduction studies have been performed in rats and rabbits. Doses up to 5 to 10 times the human dose, based on body surface area, have revealed no evidence of impaired fertility ...
-
10 OVERDOSAGEShort-Term - Acute overdosage could lead initially to hypoglycemia and subsequently to hyperglycemia. Long-Term - Long-term overdosage could result in signs and symptoms of acromegaly ...
-
11 DESCRIPTIONSEROSTIM is a human growth hormone (hGH) produced by recombinant DNA technology. SEROSTIM has 191 amino acid residues and a molecular weight of 22,125 daltons. Its amino acid sequence and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - SEROSTIM is an anabolic and anticatabolic agent which exerts its influence by interacting with specific receptors on a variety of cell types including myocytes ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies for carcinogenicity have not been performed with SEROSTIM. There is no evidence from animal studies to date of ...
-
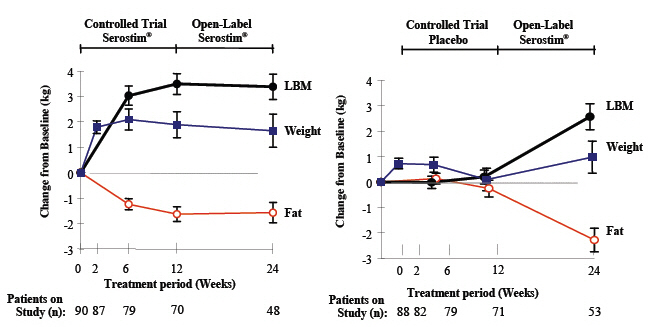
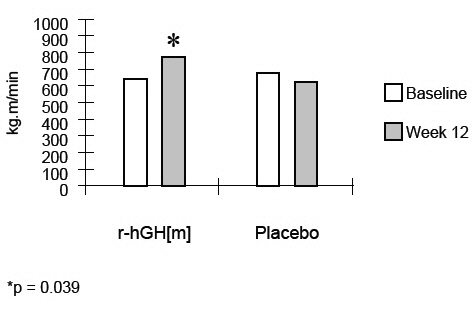
14 CLINICAL STUDIESHIV-Associated Wasting or Cachexia - The clinical efficacy of SEROSTIM in HIV-associated wasting or cachexia was assessed in two placebo-controlled trials. All study subjects received ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - SEROSTIM is available in the following forms: SEROSTIM single-use vials containing 5 mg with Sterile Water for Injection, USP. Package of 7 vials. NDC 44087-0005-7 - SEROSTIM ...
-
17 PATIENT COUNSELING INFORMATIONPatients being treated with SEROSTIM should be informed of the potential benefits and risks associated with treatment. Patients should be instructed to contact their physician should they ...
-
SPL UNCLASSIFIED SECTIONManufactured for: EMD Serono, Inc., Rockland, MA 02370
-
PRINCIPAL DISPLAY PANEL - 4 mg Kit CartonSerostim® 4 mg - (somatropin) for injection - 4 mg - For subcutaneous injection - Rx Only - 7 vials of SEROSTIM - 7 vials of Bacteriostatic Water for Injection, USP (0.9 % Benzyl Alcohol) NDC ...
-
PRINCIPAL DISPLAY PANEL - 5 mg Kit CartonSerostim® 5 mg - (somatropin) for injection - 5 mg - For subcutaneous injection - Rx Only - 7 vials SEROSTIM - 7 vials Sterile Diluent - NDC 44087-0005-7 - EMD Serono
-
PRINCIPAL DISPLAY PANEL - 6 mg Kit CartonSerostim® 6 mg - (somatropin) for injection - 6 mg - For subcutaneous injection - Rx Only - 7 vials SEROSTIM - 7 vials Sterile Diluent - NDC 44087-0006-7 - EMD Serono
-
INGREDIENTS AND APPEARANCEProduct Information