Label: SEIZALAM- midazolam hydrochloride injection, solution
- NDC Code(s): 11704-650-01, 11704-650-10
- Packager: Meridian Medical Technologies LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated January 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SEIZALAM® safely and effectively. See full prescribing information for SEIZALAM®. SEIZALAM® (midazolam injection), for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Monitor patients for respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, including SEIZALAM, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing SEIZALAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although SEIZALAM is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of SEIZALAM may precipitate acute withdrawal reactions, which can be life-threatening. For patients using SEIZALAM more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue SEIZALAM [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
SEIZALAM is indicated for the treatment of status epilepticus in adults.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose - The recommended dose of SEIZALAM is 10 mg, administered by intramuscular injection. 2.2 Important Administration Instructions - SEIZALAM should be administered by a ...
-
3 DOSAGE FORMS AND STRENGTHS
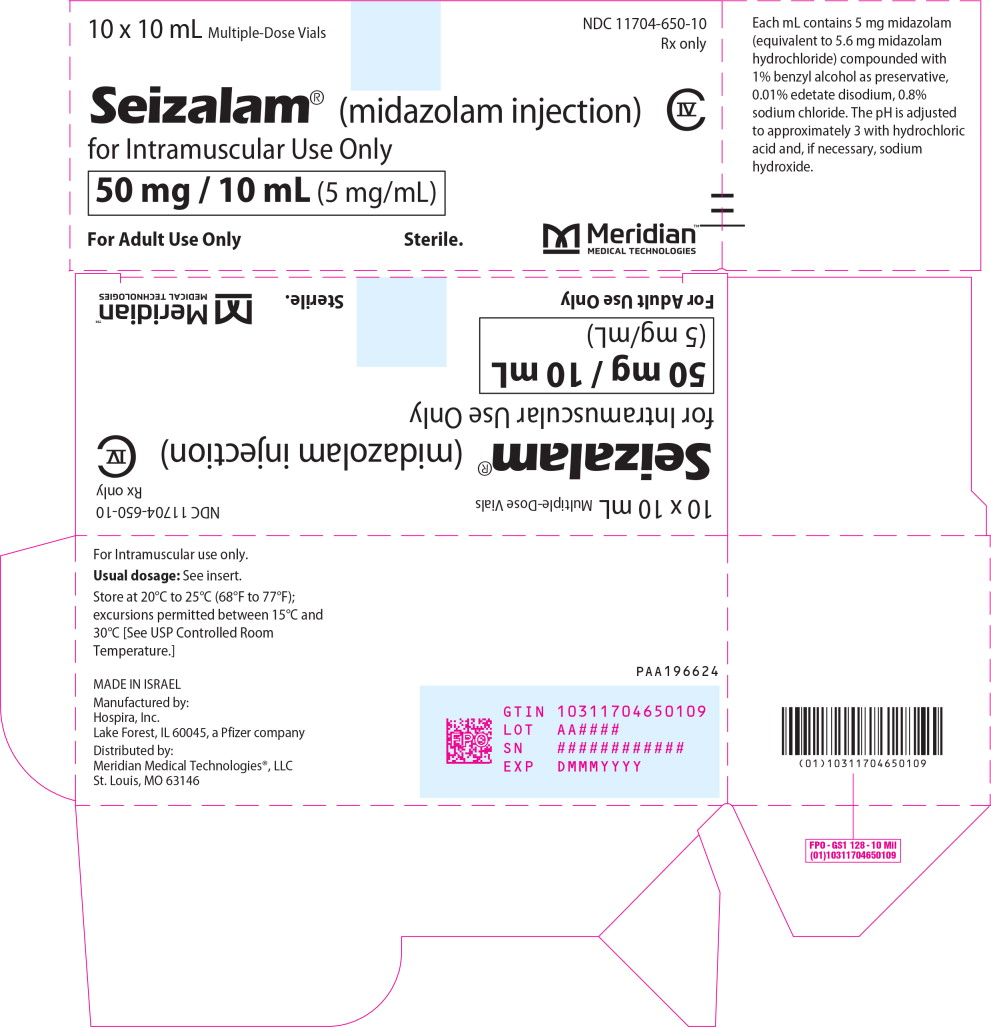
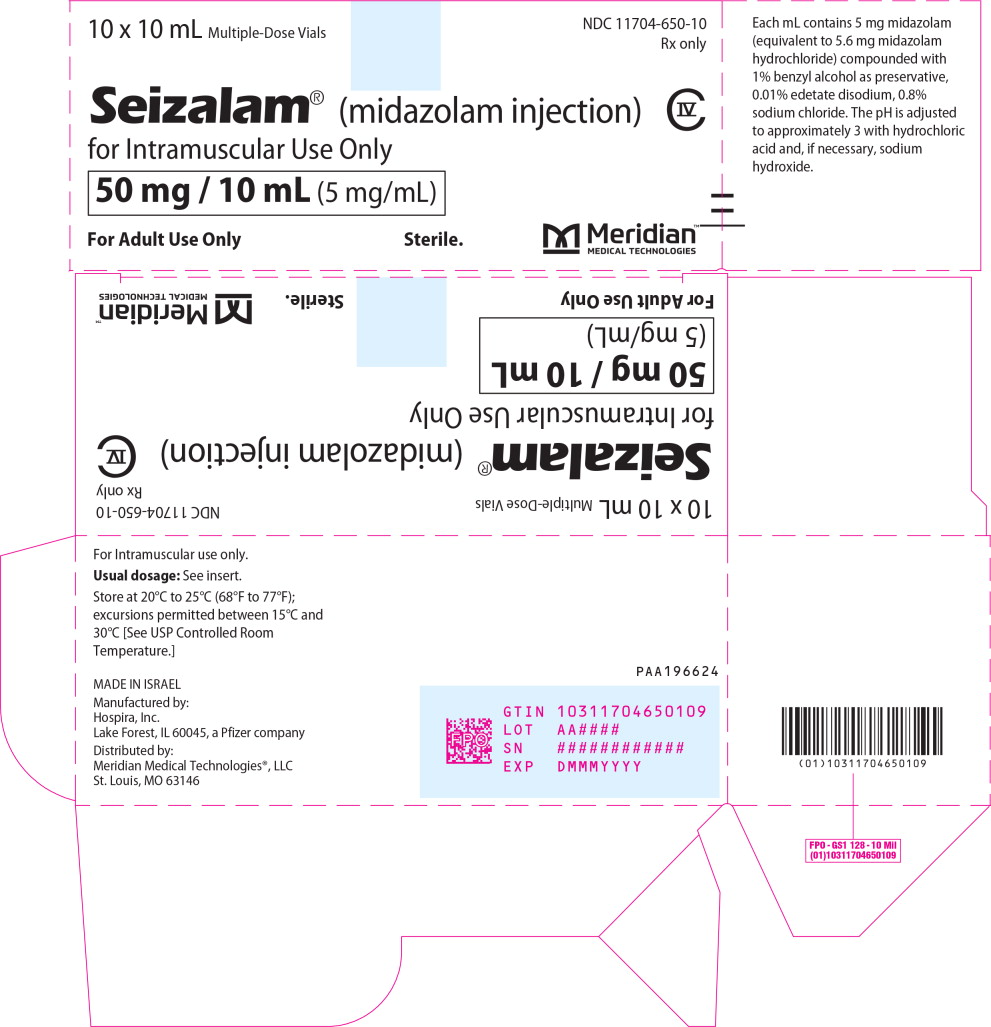
Injection: 50 mg/10 mL (5 mg/mL) of a sterile, clear, colorless to light yellow liquid solution in a multiple-dose vial.
-
4 CONTRAINDICATIONS
SEIZALAM is contraindicated in patients with a known hypersensitivity to midazolam.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids - Concomitant use of benzodiazepines, including SEIZALAM, and opioids may result in profound sedation, respiratory depression, coma, and death. If a ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections: Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)] Abuse, Misuse, and ...
-
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Use of Benzodiazepines and Opioids - The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as SEIZALAM, during ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - SEIZALAM contains midazolam hydrochloride, a Schedule IV controlled substance. 9.2 Abuse - SEIZALAM is a benzodiazepine and a CNS depressant with a potential for ...
-
10 OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion ...
-
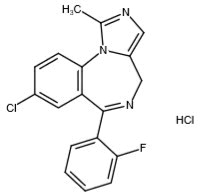
11 DESCRIPTION
Midazolam is a white to light yellow crystalline compound, insoluble in water. The hydrochloride salt of midazolam, which is formed in situ, is soluble in aqueous solutions. Chemically, midazolam ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The exact mechanism of action for midazolam in the treatment of status epilepticus is not fully understood, but is thought to involve potentiation of GABAergic ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Midazolam maleate was administered in the diet to mice and rats for 2 years at doses of 0, 1, 9, or 80 mg/kg/day ...
-
14 CLINICAL STUDIES
The safety and effectiveness of SEIZALAM for the treatment of status epilepticus was established in a multi-center, randomized, double-blind (double-dummy), active-control trial comparing ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - SEIZALAM (midazolam injection) is a clear, colorless to light yellow sterile solution available in multiple-dose fliptop vials containing 50 mg/10 mL (5 mg/mL). SEIZALAM is ...
-
17 PATIENT COUNSELING INFORMATION
Patients having seizures will likely be unresponsive or may have difficulty in comprehending counseling information. Risks from Concomitant Use with Opioids - Inform patients and caregivers ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Seizalam 5mg/mL Carton Label - 10 x 10 mL Multiple-Dose Vials - NDC 11704-650-10 - Rx only - Seizalam® (midazolam injection) for Intramuscular Use Only - 50 mg / 10 mL (5 ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Seizalam 5mg/mL Vial Label - Rx only - NDC 11704-650-01 - Seizalam® CIV - (midazolam injection) Multiple-Dose Vial for Intramuscular Use Only - 50 mg / 10 mL (5 ...
-
INGREDIENTS AND APPEARANCEProduct Information