Label: KANUMA- sebelipase alfa injection, solution, concentrate
- NDC Code(s): 25682-007-01
- Packager: Alexion Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KANUMA safely and effectively. See full prescribing information for KANUMA. KANUMA (sebelipase alfa) injection, for intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate KANUMA in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue KANUMA and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEKANUMA® is indicated for the treatment of patients with a diagnosis of Lysosomal Acid Lipase (LAL) deficiency.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommendations Prior to KANUMA Treatment - Administration of KANUMA should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including ...
-
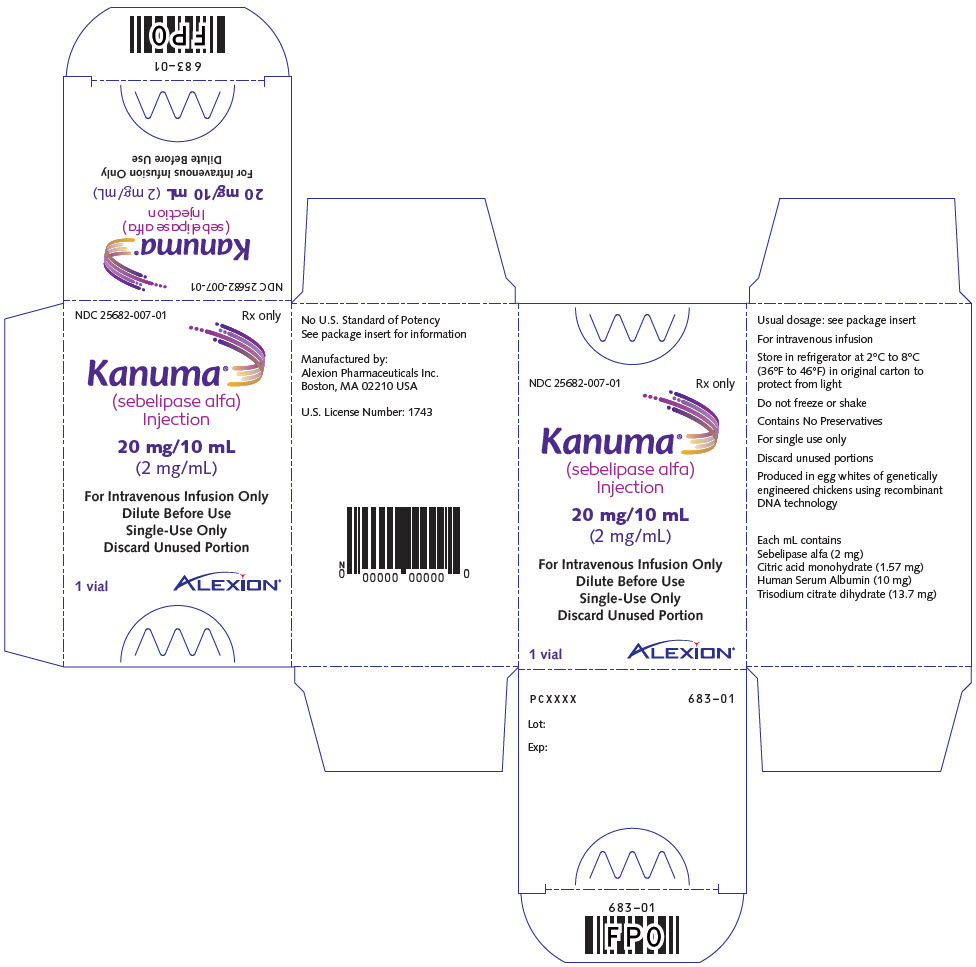
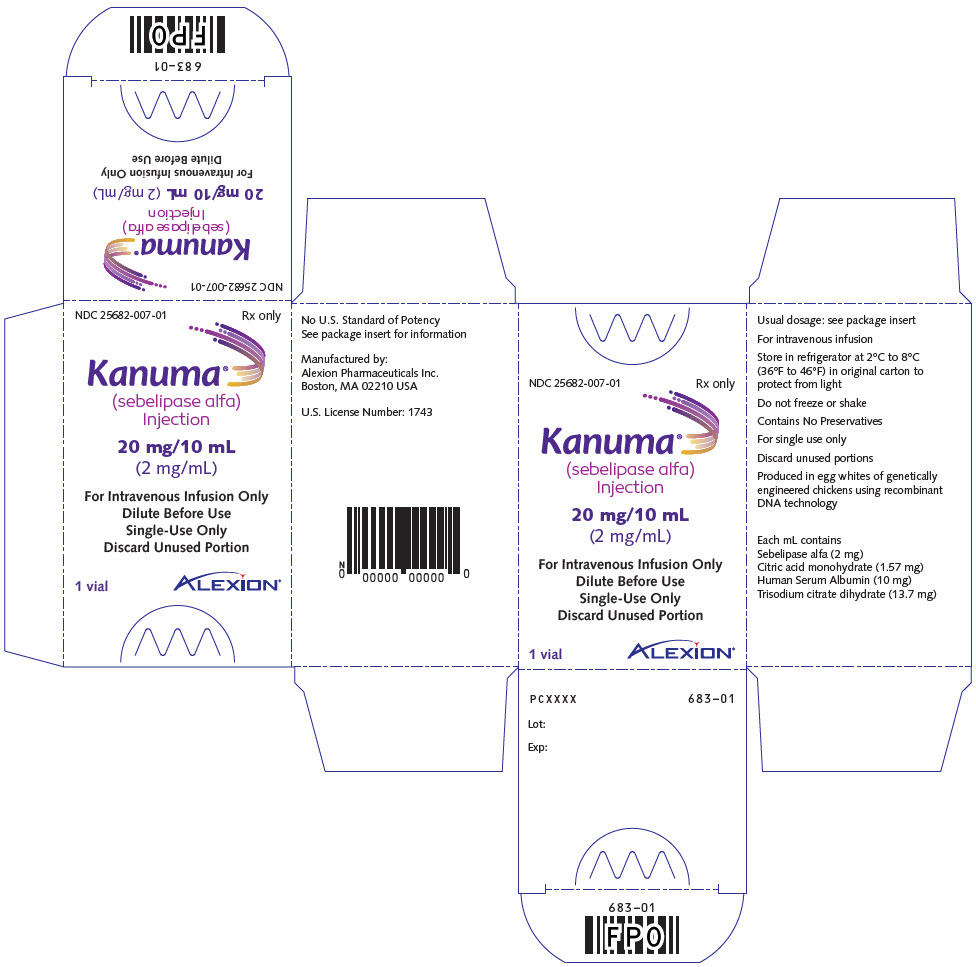
3 DOSAGE FORMS AND STRENGTHSInjection: 20 mg/10 mL (2 mg/mL) clear to slightly opalescent, colorless to slightly colored solution in single-dose vials.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions Including Anaphylaxis - Life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with enzyme replacement ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with KANUMA use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or other adverse ...
-
11 DESCRIPTIONSebelipase alfa is a recombinant human lysosomal acid lipase (rhLAL) that is a lysosomal glycoprotein enzyme produced by recombinant DNA technology in the egg white of eggs laid by genetically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - LAL deficiency is an autosomal recessive lysosomal storage disorder characterized by a genetic defect resulting in a marked decrease or loss in activity of the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with ...
-
14 CLINICAL STUDIES14.1 Infants with Rapidly Progressive LAL Deficiency Presenting within the First 6 Months of Life - A multicenter, open-label, single-arm clinical study of KANUMA was conducted in 9 infants with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKANUMA (sebelipase alfa) injection is a preservative-free, clear to slightly opalescent, colorless to slightly colored, nonpyrogenic solution supplied as 20 mg/10 mL (2 mg/mL) in single-dose ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions Including Anaphylaxis - Advise patients and caregivers that life-threatening hypersensitivity reactions, including anaphylaxis may occur with KANUMA treatment. Advise ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Alexion Pharmaceuticals, Inc. 121 Seaport Boulevard - Boston, MA 02210 USA - US License Number: 1743 - KANUMA is a registered trademark of Alexion Pharmaceuticals, Inc. © 2024 Alexion ...
-
PRINCIPAL DISPLAY PANEL - 20 mg/10 mL Vial CartonNDC 25682-007-01 - Rx only - Kanuma® (sebelipase alfa) Injection - 20 mg/10 mL - (2 mg/mL) For Intravenous Infusion Only - Dilute Before Use - Single-Use Only - Discard Unused Portion - 1 vial - ALEXION®
-
INGREDIENTS AND APPEARANCEProduct Information