Label: SEYSARA- sarecycline hydrochloride tablet, coated
- NDC Code(s): 16110-245-30, 16110-246-30, 16110-247-30
- Packager: Almirall, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SEYSARA® safely and effectively. See full prescribing information for SEYSARA®. SEYSARA® (sarecycline) tablets for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESEYSARA® (sarecycline) tablet, is indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older. Limitations of ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of SEYSARA is based on body weight described in Table 1. If there is no improvement after 12 weeks, reassess treatment with SEYSARA. Table 1: Dosing Table for ...
-
3 DOSAGE FORMS AND STRENGTHSSEYSARA (sarecycline) tablets: 60 mg: capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side. 100 mg: capsule-shaped, yellow, film-coated tablets ...
-
4 CONTRAINDICATIONSSEYSARA is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
5 WARNINGS AND PRECAUTIONS5.1 Teratogenic Effects - SEYSARA, like other tetracyclines, can cause fetal harm when administered to a pregnant woman. If SEYSARA is used during pregnancy or if the patient becomes pregnant ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on SEYSARA - Oral Retinoids - Tetracyclines may cause increased intracranial pressure as do oral retinoids, including isotretinoin and acitretin [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - SEYSARA, like tetracycline class drugs, may cause fetal harm, permanent discoloration of teeth, and reversible inhibition of bone growth when administered during ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases ...
-
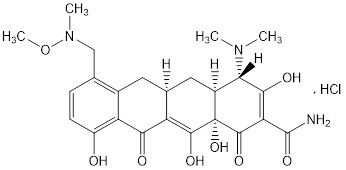
11 DESCRIPTIONSEYSARA (sarecycline) tablets are a tetracycline class drug for oral administration. Sarecycline hydrochloride is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sarecycline is an aminomethylcycline within the tetracycline class of drugs. [see CLINICAL PHARMACOLOGY (12.4)]. The mechanism of action of SEYSARA in treating the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year oral mouse carcinogenicity study and a 2-year oral rat carcinogenicity study, no drug-related neoplasms were observed in ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily SEYSARA was assessed in two 12-week multicenter, randomized, double-blind, placebo-controlled studies (Study 1 [NCT02320149] and Study 2 [NCT02322866]) ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - 1) SEYSARA (sarecycline) tablets, 60 mg are capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side. Bottles of 30 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients taking SEYSARA should receive the following information and instructions: SEYSARA should not be used ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 07/2019 - PATIENT INFORMATION - SEYSARA® (SAY' sara) (sarecycline ...
-
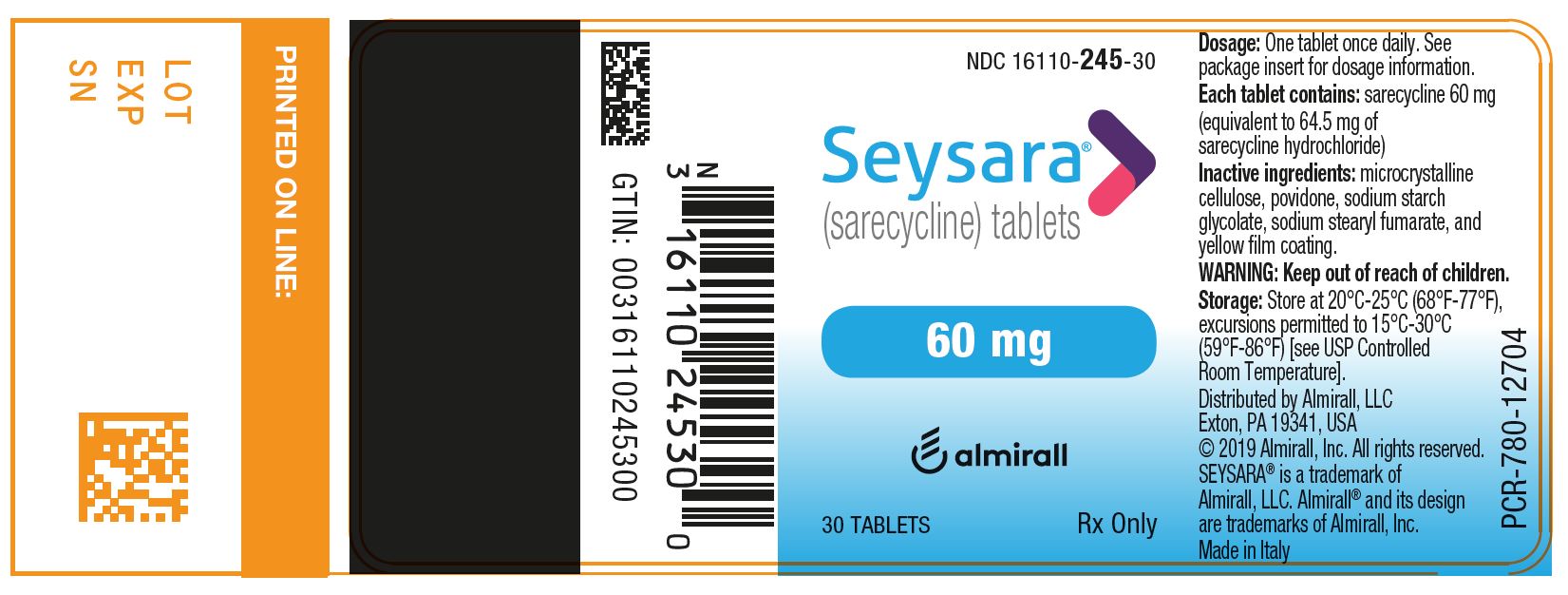
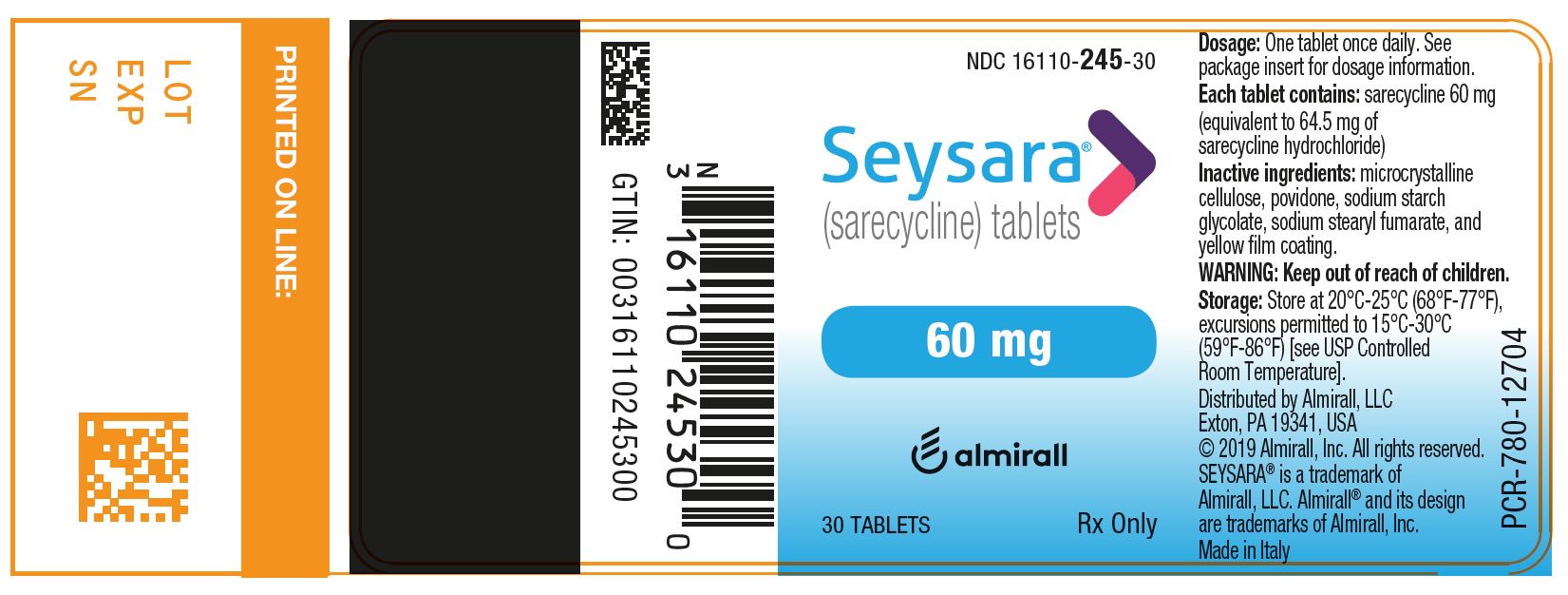
PRINCIPAL DISPLAY PANEL - NDC: 16110-245-30 - 60 mg 30-count Bottle Label
-
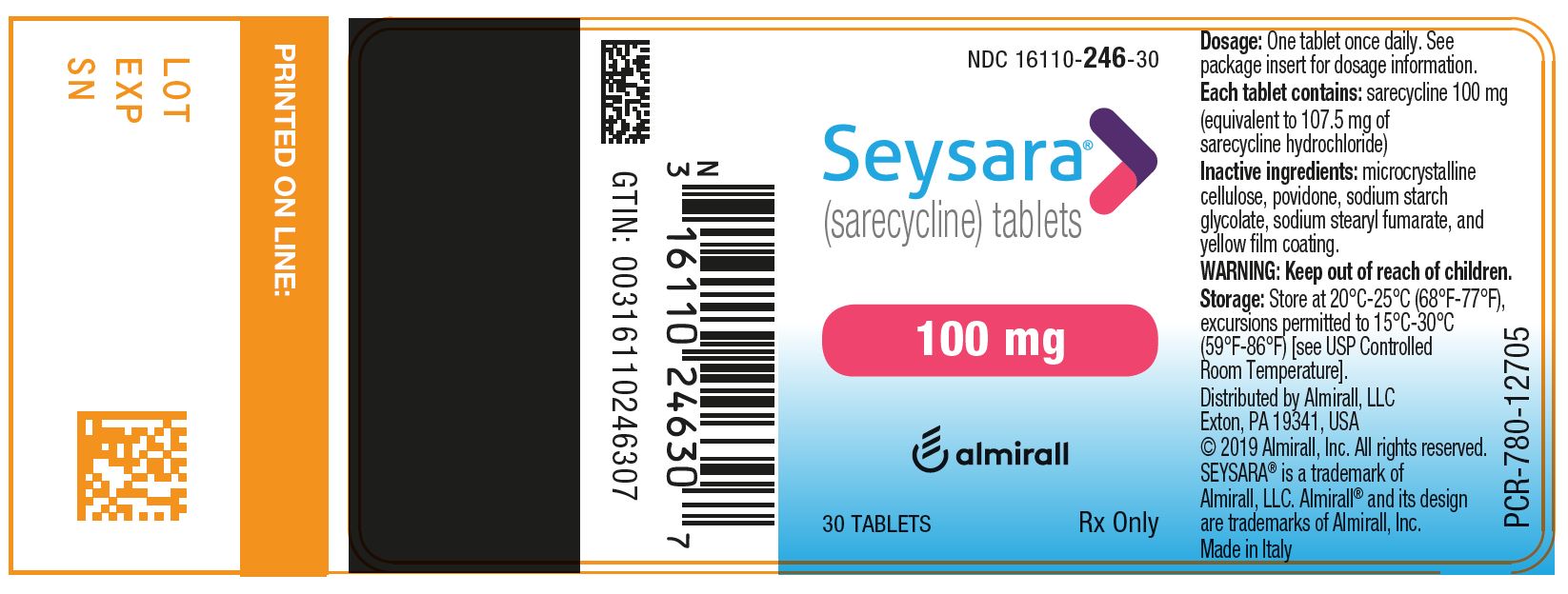
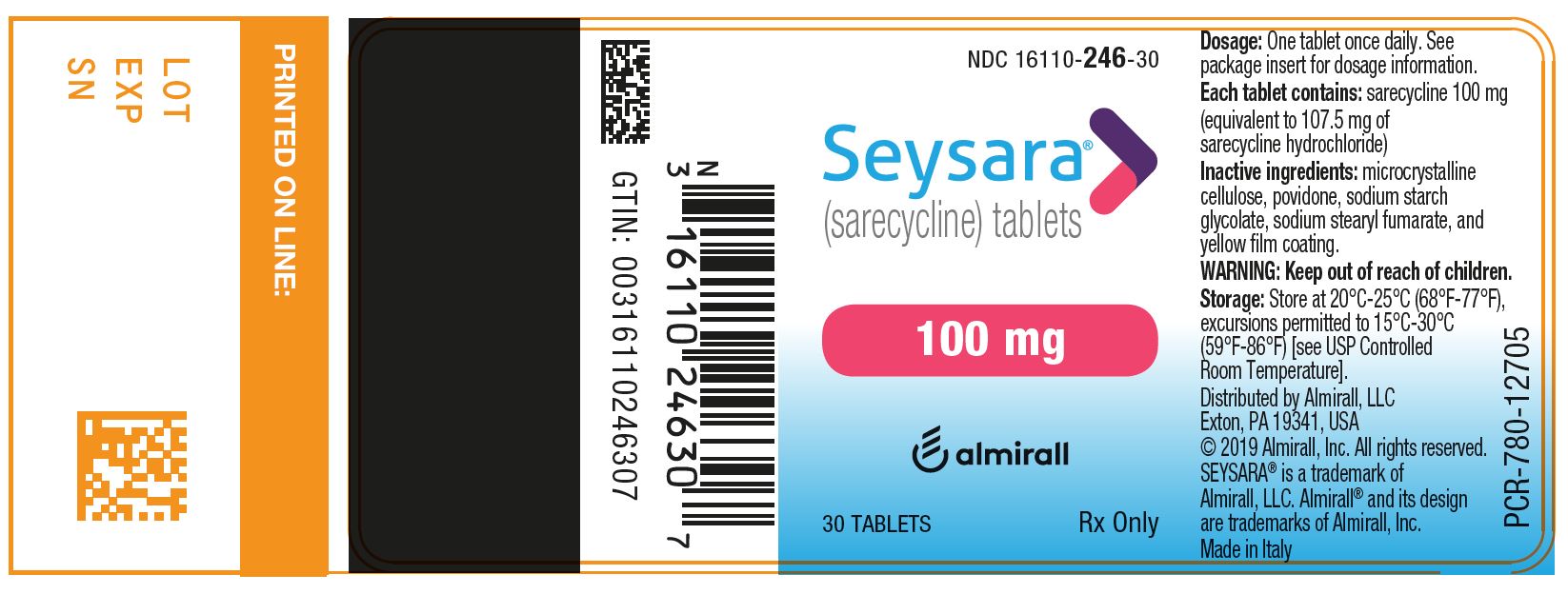
PRINCIPAL DISPLAY PANEL - NDC: 16110-246-30 - 100 mg 30-count Bottle Label
-
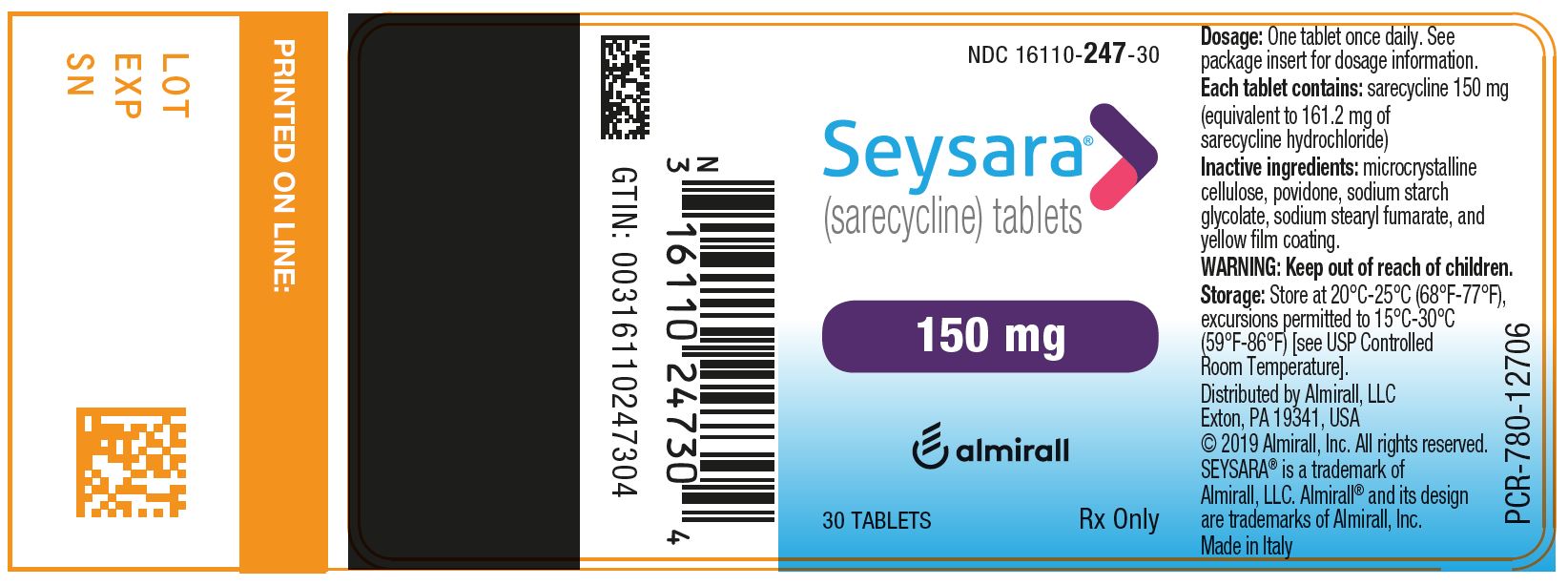
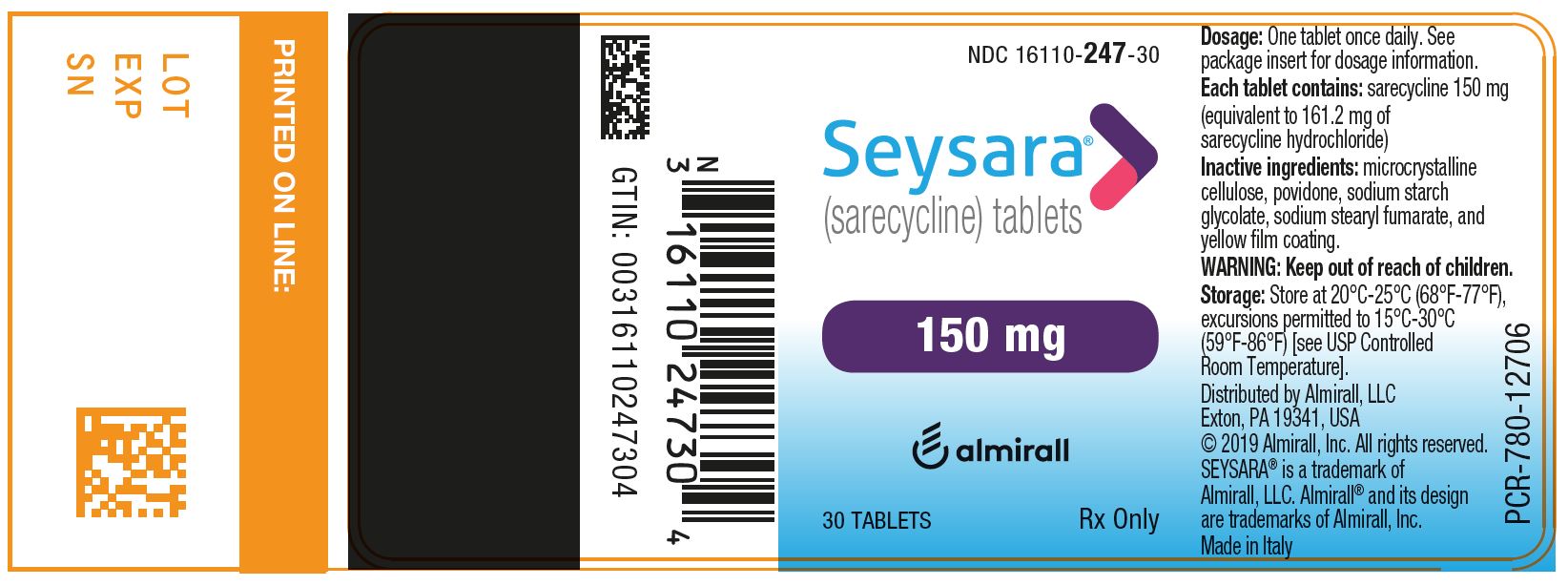
PRINCIPAL DISPLAY PANEL - NDC: 16110-247-30 - 150 mg 30-count Bottle Label
-
INGREDIENTS AND APPEARANCEProduct Information