Label: SALAGEN- pilocarpine hydrochloride tablet, film coated
- NDC Code(s): 59212-705-10, 59212-775-10
- Packager: Advanz Pharma (US) Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
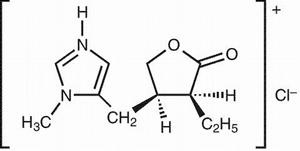
DESCRIPTIONSALAGEN® Tablets contain pilocarpine hydrochloride, a cholinergic agonist for oral use. Pilocarpine hydrochloride is a hygroscopic, odorless, bitter tasting white crystal or powder which is ...
-
CLINICAL PHARMACOLOGYPharmacodynamics: Pilocarpine is a cholinergic parasympathomimetic agent exerting a broad spectrum of pharmacologic effects with predominant muscarinic action. Pilocarpine, in appropriate ...
-
CLINICAL STUDIESHead & Neck Cancer Patients: A 12 week randomized, double-blind, placebo-controlled study in 207 patients (142 men, 65 women) was conducted in patients whose mean age was 58.5 years with a range ...
-
INDICATIONS & USAGESALAGEN® Tablets are indicated for 1) the treatment of symptoms of dry mouth from salivary gland hypofunction caused by radiotherapy for cancer of the head and neck; and 2) the treatment of ...
-
CONTRAINDICATIONSSALAGEN® Tablets are contraindicated in patients with uncontrolled asthma, known hypersensitivity to pilocarpine, and when miosis is undesirable, e.g., in acute iritis and in narrow-angle (angle ...
-
WARNINGSCardiovascular Disease: Patients with significant cardiovascular disease may be unable to compensate for transient changes in hemodynamics or rhythm induced by pilocarpine. Pulmonary edema has ...
-
PRECAUTIONSGeneral - Pilocarpine toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory ...
-
ADVERSE REACTIONSHead & Neck Cancer Patients: In controlled studies, 217 patients received pilocarpine, of whom 68% were men and 32% were women. Race distribution was 91% Caucasian, 8% Black, and 1% of other ...
-
MANAGEMENT OF OVERDOSEFatal overdosage with pilocarpine has been reported in the scientific literature at doses presumed to be greater than 100 mg in two hospitalized patients. 100 mg of pilocarpine is considered ...
-
DOSAGE AND ADMINISTRATIONRegardless of the indication, the starting dose in patients with moderate hepatic impairment should be 5 mg twice daily, followed by adjustment based on therapeutic response and tolerability ...
-
HOW SUPPLIEDSALAGEN® Tablets, 5 mg, are white, film coated, debossed round tablets, coded SAL 5. Each tablet contains 5 mg pilocarpine hydrochloride. They are supplied as follows: NDC 59212-705-10 bottles of ...
-
PRINCIPAL DISPLAY PANELNDA 59212-705-10 5 mg - SALAGEN® Tablets(pilocarpine hydrochloride) Rx only - 100 film coated tablets - 5 mg each - ADVANZ PHARMA
-
PRINCIPAL DISPLAY PANELNDA 59212-775-10 7.5 mg - SALAGEN® Tablets(pilocarpine hydrochloride) Rx only - 100 film coated tablets - 7.5 mg each - ADVANZ PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information