Label: RYANODEX DANTROLENE SODIUM- dantrolene sodium injection, suspension
- NDC Code(s): 42367-540-32
- Packager: Eagle Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RYANODEX safely and effectively. See full prescribing information for RYANODEX. RYANODEX® (dantrolene sodium) for injectable ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERYANODEX® is indicated for the: Treatment of malignant hyperthermia in conjunction with appropriate supportive measures [see Dosage and Administration (2.1)] Prevention of malignant hyperthermia ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage for Treatment of Malignant Hyperthermia - In addition to RYANODEX treatment, institute the following supportive measures: Discontinue use of malignant hyperthermia (MH)-triggering ...
-
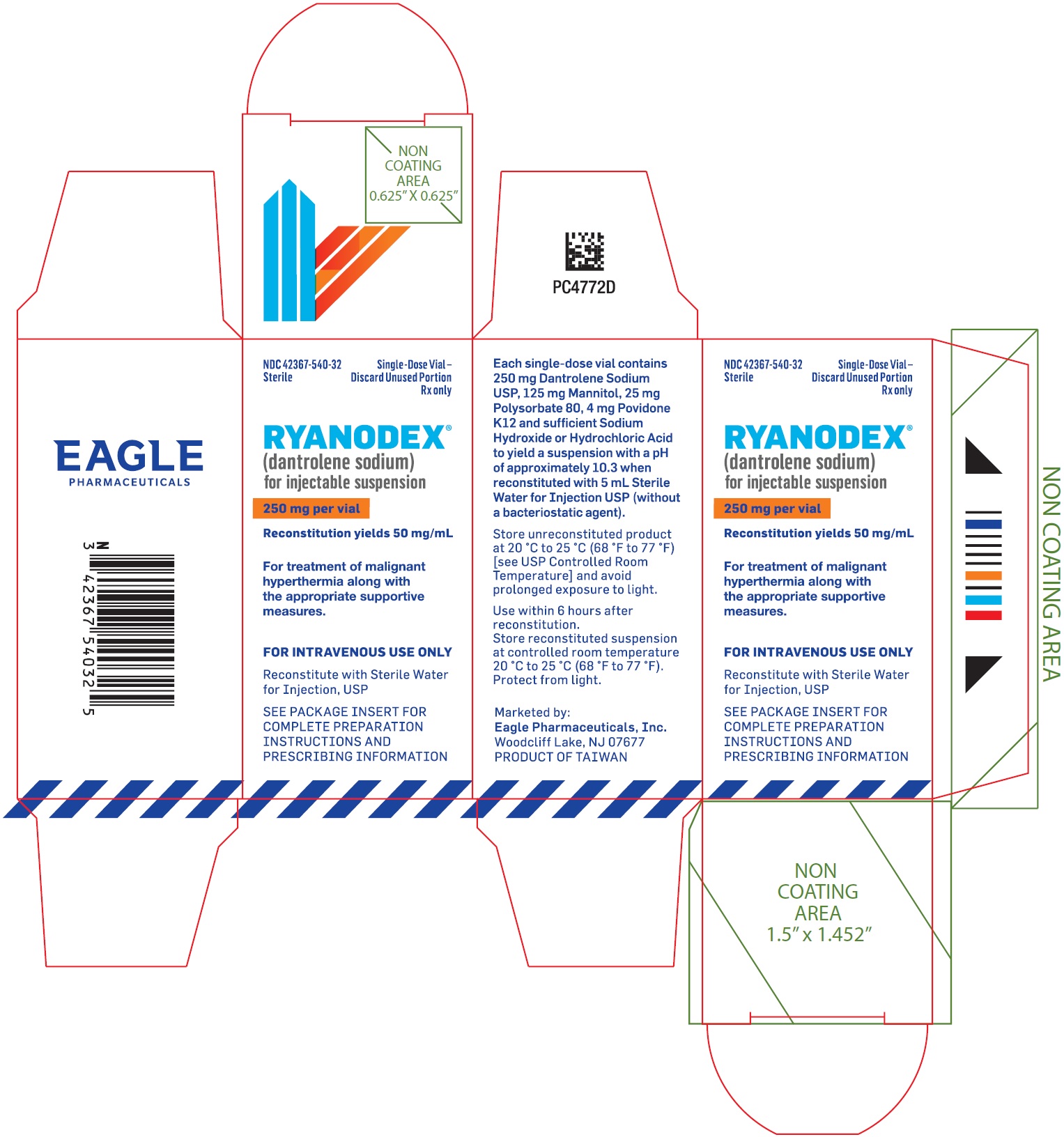
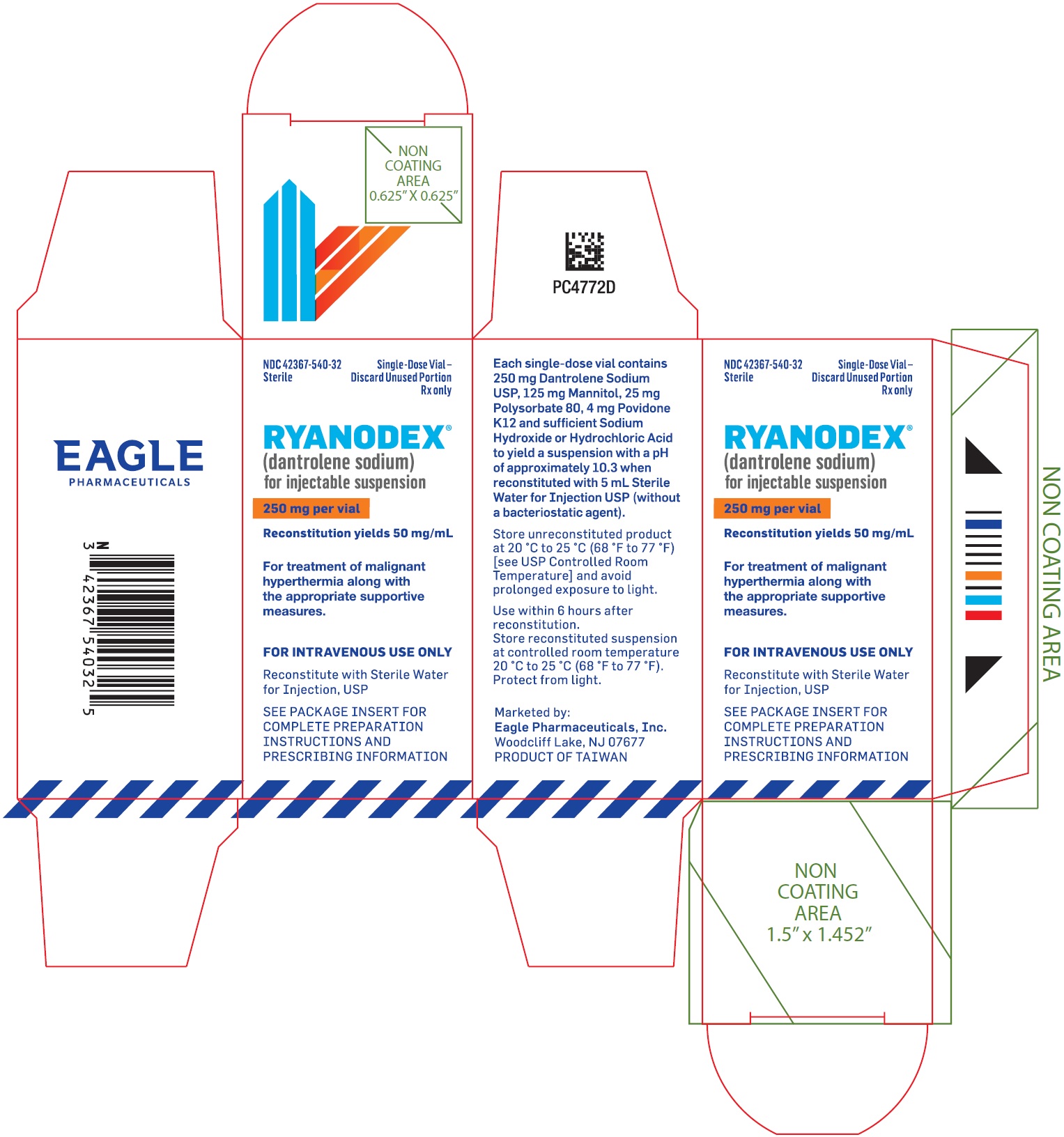
3 DOSAGE FORMS AND STRENGTHSFor injectable suspension: RYANODEX is a sterile, lyophilized powder containing 250 mg of dantrolene sodium for reconstitution, in single-dose vials
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Muscle Weakness - RYANODEX is associated with skeletal muscle weakness. The administration of RYANODEX in human volunteers has been associated with loss of grip strength and weakness in the ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Calcium Channel Blockers - Cardiovascular collapse in association with marked hyperkalemia has been reported in patients receiving dantrolene in combination with calcium channel blockers. The ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published case reports with RYANODEX use in pregnant women are insufficient to evaluate for a drug-associated ...
-
10 OVERDOSAGE10.1 Overdosage Symptoms - Overdosage symptoms include, but are not limited to, muscular weakness and alterations in the state of consciousness (e.g., lethargy, coma), vomiting, diarrhea, and ...
-
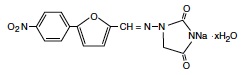
11 DESCRIPTIONRYANODEX® (dantrolene sodium) for injectable suspension is a sterile lyophilized powder. RYANODEX is supplied in 20 mL vials containing 250 mg dantrolene sodium and the following inactive ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In isolated nerve-muscle preparation, dantrolene has been shown to produce relaxation by affecting the contractile response of the muscle at a site beyond the myoneural ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Sprague-Dawley female rats fed dantrolene sodium for 18 months at dosage levels of 15, 30, and 60 mg/kg/day showed ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRYANODEX® (NDC 42367-540-32) is available in 20 mL vials containing a sterile lyophilized mixture of 250 mg dantrolene sodium for reconstitution with 5 mL sterile water for injection USP (without ...
-
17 PATIENT COUNSELING INFORMATIONInform patients, their families, or their caregivers of the following: Muscle Weakness - Muscle weakness (i.e., decrease in grip strength and weakness of leg muscles ...
-
PRINCIPAL DISPLAY PANEL - NDC: 42367-540-32 - Carton LabelNDC 42367-540-32 - Sterile - Single Use Only - Discard Unused Portion - Rx only - Ryanodex® (dantrolene sodium ...
-
PRINCIPAL DISPLAY PANEL - NDC: 42367-540-32 - Vial LabelNDC 42367-540-32 - Sterile - Single Use Only - Discard Unused Portion - Rx only - Ryanodex® (dantrolene sodium ...
-
INGREDIENTS AND APPEARANCEProduct Information