Label: RUXIENCE- rituximab-pvvr injection, solution
- NDC Code(s): 0069-0238-01, 0069-0249-01
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RUXIENCE safely and effectively. See full prescribing information for RUXIENCE. RUXIENCE® (rituximab-pvvr) injection, for ...These highlights do not include all the information needed to use RUXIENCE safely and effectively. See full prescribing information for RUXIENCE.
RUXIENCE® (rituximab-pvvr) injection, for intravenous use
Initial U.S. Approval: 2019
RUXIENCE (rituximab-pvvr) is biosimilar* to RITUXAN (rituximab)WARNING: FATAL INFUSION-RELATED REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete boxed warning.
- •
- Fatal infusion-related reactions within 24 hours of rituximab infusion; approximately 80% of fatal reactions occurred with first infusion. Monitor patients and discontinue RUXIENCE infusion for severe reactions (5.1, 6.1).
- •
- Severe mucocutaneous reactions, some with fatal outcomes (5.2).
- •
- Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death (5.3).
- •
- Progressive multifocal leukoencephalopathy (PML) resulting in death (5.4, 6.3).
RECENT MAJOR CHANGES
Dosage and Administration, Administration and Storage (2.8)
10/2023
INDICATIONS AND USAGE
RUXIENCE is a CD20-directed cytolytic antibody indicated for the treatment of:
- •
- Adult patients with Non-Hodgkin's Lymphoma (NHL) (1.1).
- o
- Relapsed or refractory, low grade or follicular, CD20-positive B-cell NHL as a single agent.
- o
- Previously untreated follicular, CD20-positive, B-cell NHL in combination with first line chemotherapy and, in patients achieving a complete or partial response to a rituximab product in combination with chemotherapy, as single-agent maintenance therapy.
- o
- Non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line cyclophosphamide, vincristine, and prednisone (CVP) chemotherapy.
- o
- Previously untreated diffuse large B-cell, CD20-positive NHL in combination with (cyclophosphamide, doxorubicin, vincristine, and prednisone) (CHOP) or other anthracycline-based chemotherapy regimens.
- •
- Adult patients with Chronic Lymphocytic Leukemia (CLL) (1.2).
- o
- Previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide (FC).
- •
- Rheumatoid Arthritis (RA) in combination with methotrexate in adult patients with moderately-to severely-active RA who have inadequate response to one or more TNF antagonist therapies (1.3).
- •
- Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA) in adult patients in combination with glucocorticoids (1.4).
DOSAGE AND ADMINISTRATION
- •
- Administer only as an intravenous infusion (2.1).
- •
- Do not administer as an intravenous push or bolus (2.1).
- •
- RUXIENCE should only be administered by a healthcare professional with appropriate medical support to manage severe infusion-related reactions that can be fatal if they occur (2.1).
- •
- The dose for adult B-cell NHL is 375 mg/m2 (2.2).
- •
- The dose for CLL is 375 mg/m2 in the first cycle and 500 mg/m2 in Cycles 2–6, in combination with FC, administered every 28 days (2.3).
- •
- The dose as a component of Zevalin® (ibritumomab tiuxetan) Therapeutic Regimen is 250 mg/m2 (2.4).
- •
- The dose for RA in combination with methotrexate is two-1,000 mg intravenous infusions separated by 2 weeks (one course) every 24 weeks or based on clinical evaluation, but not sooner than every 16 weeks. Methylprednisolone 100 mg intravenous or equivalent glucocorticoid is recommended 30 minutes prior to each infusion (2.5).
- •
- The induction dose for adult patients with active GPA and MPA in combination with glucocorticoids is 375 mg/m2 once weekly for 4 weeks. The follow up dose for adult patients with GPA and MPA who have achieved disease control with induction treatment, in combination with glucocorticoids is two 500 mg intravenous infusions separated by two weeks, followed by a 500 mg intravenous infusion every 6 months thereafter based on clinical evaluation (2.6).
DOSAGE FORMS AND STRENGTHS
- •
- Injection: 100 mg/10 mL (10 mg/mL) and 500 mg/50 mL (10 mg/mL) solution in single-dose vials (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- •
- Tumor lysis syndrome: Administer aggressive intravenous hydration, anti-hyperuricemic agents, monitor renal function (5.5).
- •
- Infections: Withhold RUXIENCE and institute appropriate anti-infective therapy (5.6).
- •
- Cardiac adverse reactions: Discontinue infusions in case of serious or life-threatening events (5.7).
- •
- Renal toxicity: Discontinue in patients with rising serum creatinine or oliguria (5.8).
- •
- Bowel obstruction and perforation: Consider and evaluate for abdominal pain, vomiting, or related symptoms (5.9).
- •
- Immunizations: Live virus vaccinations prior to or during RUXIENCE treatment are not recommended (5.10).
- •
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception (5.11).
ADVERSE REACTIONS
Most common adverse reactions in clinical trials were:
- •
- NHL (greater than or equal to 25%): infusion-related reactions, fever, lymphopenia, chills, infection and asthenia (6.1).
- •
- CLL (greater than or equal to 25%): infusion-related reactions and neutropenia (6.1).
- •
- RA (greater than or equal to 10%): upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis (other important adverse reactions include infusion-related reactions, serious infections, and cardiovascular events) (6.1).
- •
- GPA and MPA (greater than or equal to 15%): infections, nausea, diarrhea, headache, muscle spasms, anemia, peripheral edema, infusion-related reactions (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Renal toxicity when used in combination with cisplatin (5.8).
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
- *
- Biosimilar means that the biological product is approved based on data demonstrating that it is highly similar to an FDA-approved biological product, known as a reference product, and that there are no clinically meaningful differences between the biosimilar product and the reference product. Biosimilarity of RUXIENCE has been demonstrated for the condition(s) of use (e.g., indication(s), dosing regimen(s)), strength(s), dosage form(s), and route(s) of administration described in its Full Prescribing Information.
Revised: 10/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FATAL INFUSION-RELATED REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
1 INDICATIONS AND USAGE
1.1 Non-Hodgkin's Lymphoma (NHL)
1.2 Chronic Lymphocytic Leukemia (CLL)
1.3 Rheumatoid Arthritis (RA)
1.4 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dose for Non-Hodgkin's Lymphoma (NHL)
2.3 Recommended Dose for Chronic Lymphocytic Leukemia (CLL)
2.4 Recommended Dose as a Component of Zevalin® for Treatment of NHL
2.5 Recommended Dose for Rheumatoid Arthritis (RA)
2.6 Recommended Dose for Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
2.7 Recommended Dose for Premedication and Prophylactic Medications
2.8 Administration and Storage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
5.2 Severe Mucocutaneous Reactions
5.3 Hepatitis B Virus (HBV) Reactivation
5.4 Progressive Multifocal Leukoencephalopathy (PML)
5.5 Tumor Lysis Syndrome (TLS)
5.6 Infections
5.7 Cardiovascular Adverse Reactions
5.8 Renal Toxicity
5.9 Bowel Obstruction and Perforation
5.10 Immunization
5.11 Embryo-Fetal Toxicity
5.12 Concomitant Use with Other Biologic Agents and DMARDS other than Methotrexate in RA, GPA and MPA
5.13 Use in RA Patients Who Have Not Had Prior Inadequate Response to Tumor Necrosis Factor (TNF) Antagonists
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
14.2 Previously Untreated, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
14.3 Diffuse Large B-Cell NHL (DLBCL)
14.4 Ninety-Minute Infusions in Previously Untreated Follicular NHL and DLBCL
14.5 Chronic Lymphocytic Leukemia (CLL)
14.6 Rheumatoid Arthritis (RA)
14.7 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)Infusion-Related Reactions - Administration of rituximab products can result in serious, including fatal, infusion-related reactions. Deaths within 24 hours of rituximab infusion have occurred ...
WARNING: FATAL INFUSION-RELATED REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
Infusion-Related Reactions
Administration of rituximab products can result in serious, including fatal, infusion-related reactions. Deaths within 24 hours of rituximab infusion have occurred. Approximately 80% of fatal infusion-related reactions occurred in association with the first infusion. Monitor patients closely. Discontinue RUXIENCE infusion for severe reactions and provide medical treatment for Grade 3 or 4 infusion-related reactions [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
Severe Mucocutaneous Reactions
Severe, including fatal, mucocutaneous reactions can occur in patients receiving rituximab products [see Warnings and Precautions (5.2)].
Hepatitis B Virus (HBV) Reactivation
HBV reactivation can occur in patients treated with rituximab products, in some cases resulting in fulminant hepatitis, hepatic failure, and death. Screen all patients for HBV infection before treatment initiation, and monitor patients during and after treatment with RUXIENCE. Discontinue RUXIENCE and concomitant medications in the event of HBV reactivation [see Warnings and Precautions (5.3)].
CloseProgressive Multifocal Leukoencephalopathy (PML)
Progressive Multifocal Leukoencephalopathy (PML), including fatal PML, can occur in patients receiving rituximab products [see Warnings and Precautions (5.4), Adverse Reactions (6.3)].
-
1 INDICATIONS AND USAGE1.1 Non-Hodgkin's Lymphoma (NHL) RUXIENCE is indicated for the treatment of adult patients with: • Relapsed or refractory, low-grade or follicular, CD20-positive, B-cell NHL as a single ...
1.1 Non-Hodgkin's Lymphoma (NHL)
RUXIENCE is indicated for the treatment of adult patients with:
- •
- Relapsed or refractory, low-grade or follicular, CD20-positive, B-cell NHL as a single agent.
- •
- Previously untreated follicular, CD20-positive, B-cell NHL in combination with first line chemotherapy and, in patients achieving a complete or partial response to a rituximab product in combination with chemotherapy, as single-agent maintenance therapy.
- •
- Non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line cyclophosphamide, vincristine, and prednisone (CVP) chemotherapy.
- •
- Previously untreated diffuse large B-cell, CD20-positive NHL in combination with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
1.2 Chronic Lymphocytic Leukemia (CLL)
RUXIENCE, in combination with fludarabine and cyclophosphamide (FC), is indicated for the treatment of adult patients with previously untreated and previously treated CD20-positive CLL.
1.3 Rheumatoid Arthritis (RA)
RUXIENCE, in combination with methotrexate, is indicated for the treatment of adult patients with moderately-to severely-active rheumatoid arthritis who have had an inadequate response to one or more Tumor Necrosis Factor (TNF) antagonist therapies.
Close1.4 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
RUXIENCE, in combination with glucocorticoids, is indicated for the treatment of adult patients with Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA).
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosing Information - Administer only as an intravenous infusion [see Dosage and Administration (2.8)]. Do not administer as an intravenous push or bolus. RUXIENCE should only be ...
2.1 Important Dosing Information
Administer only as an intravenous infusion [see Dosage and Administration (2.8)]. Do not administer as an intravenous push or bolus.
RUXIENCE should only be administered by a healthcare professional with appropriate medical support to manage severe infusion-related reactions that can be fatal if they occur [see Warnings and Precautions (5.1)].
Premedicate before each infusion [see Dosage and Administration (2.7)].
Prior to First Infusion
Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with RUXIENCE [see Warnings and Precautions (5.3)]. Obtain complete blood counts (CBC) including platelets prior to the first dose.
During RUXIENCE Therapy
In patients with lymphoid malignancies, during treatment with RUXIENCE monotherapy, obtain complete blood counts (CBC) with differential and platelet counts prior to each RUXIENCE course. During treatment with RUXIENCE and chemotherapy, obtain CBC with differential and platelet counts at weekly to monthly intervals and more frequently in patients who develop cytopenias [see Adverse Reactions (6.1)]. In patients with RA, GPA or MPA, obtain CBC with differential and platelet counts at two to four month intervals during RUXIENCE therapy. Continue to monitor for cytopenias after final dose and until resolution.
- •
-
First Infusion
Standard Infusion: Initiate infusion at a rate of 50 mg/hour. In the absence of infusion toxicity, increase infusion rate by 50 mg/hour increments every 30 minutes, to a maximum of 400 mg/hour.
- •
-
Subsequent Infusions
Standard Infusion: Initiate infusion at a rate of 100 mg/hour. In the absence of infusion toxicity, increase rate by 100 mg/hour increments at 30-minute intervals, to a maximum of 400 mg/hour.
For Previously Untreated Follicular NHL and DLBCL Adult Patients: If patients did not experience a Grade 3 or 4 infusion-related adverse event during Cycle 1, a 90-minute infusion can be administered in Cycle 2 with a glucocorticoid-containing chemotherapy regimen.
Initiate at a rate of 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes. If the 90-minute infusion is tolerated in Cycle 2, the same rate can be used when administering the remainder of the treatment regimen (through Cycle 6 or 8).
Patients who have clinically significant cardiovascular disease or who have a circulating lymphocyte count greater than or equal to 5,000/mm3 before Cycle 2 should not be administered the 90-minute infusion [see Clinical Studies (14.4)]. - •
- Interrupt the infusion or slow the infusion rate for infusion-related reactions [see Boxed Warning, Warnings and Precautions (5.1)]. Continue the infusion at one-half the previous rate upon improvement of symptoms.
2.2 Recommended Dose for Non-Hodgkin's Lymphoma (NHL)
The recommended dose is 375 mg/m2 as an intravenous infusion according to the following schedules:
- •
-
Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
Administer once weekly for 4 or 8 doses. - •
-
Retreatment for Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
Administer once weekly for 4 doses. - •
-
Previously Untreated, Follicular, CD20-Positive, B-Cell NHL
Administer on Day 1 of each cycle of chemotherapy for up to 8 doses. In patients with complete or partial response, initiate RUXIENCE maintenance eight weeks following completion of a rituximab product in combination with chemotherapy. Administer RUXIENCE as a single agent every 8 weeks for 12 doses. - •
-
Non-progressing, Low-Grade, CD20-Positive, B-Cell NHL, after first-line CVP chemotherapy
Following completion of 6–8 cycles of CVP chemotherapy, administer once weekly for 4 doses at 6-month intervals to a maximum of 16 doses. - •
-
Diffuse Large B-Cell NHL
Administer on Day 1 of each cycle of chemotherapy for up to 8 infusions.
2.3 Recommended Dose for Chronic Lymphocytic Leukemia (CLL)
The recommended dose is 375 mg/m2 the day prior to the initiation of FC chemotherapy, then 500 mg/m2 on Day 1 of Cycles 2–6 (every 28 days).
2.4 Recommended Dose as a Component of Zevalin® for Treatment of NHL
When used as part of the Zevalin therapeutic regimen, infuse 250 mg/m2 in accordance with the Zevalin package insert. Refer to the Zevalin package insert for full prescribing information regarding the Zevalin therapeutic regimen.
2.5 Recommended Dose for Rheumatoid Arthritis (RA)
- •
- Administer RUXIENCE as two-1,000 mg intravenous infusions separated by 2 weeks.
- •
- Glucocorticoids administered as methylprednisolone 100 mg intravenous or its equivalent 30 minutes prior to each infusion are recommended to reduce the incidence and severity of infusion-related reactions.
- •
- Subsequent courses should be administered every 24 weeks or based on clinical evaluation, but not sooner than every 16 weeks.
- •
- RUXIENCE is given in combination with methotrexate.
2.6 Recommended Dose for Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Induction Treatment of Adult Patients with Active GPA/MPA
- •
- Administer RUXIENCE as a 375 mg/m2 intravenous infusion once weekly for 4 weeks for patients with active GPA or MPA.
- •
- Glucocorticoids administered as methylprednisolone 1,000 mg intravenously per day for 1 to 3 days followed by oral prednisone as per clinical practice. This regimen should begin within 14 days prior to or with the initiation of RUXIENCE and may continue during and after the 4 week induction course of RUXIENCE treatment.
Follow up Treatment of Adult Patients with GPA/MPA who have Achieved Disease Control with Induction Treatment
- •
- Administer RUXIENCE as two 500 mg intravenous infusions separated by two weeks, followed by a 500 mg intravenous infusion every 6 months thereafter based on clinical evaluation.
- •
- If induction treatment of active disease was with a rituximab product, initiate follow up treatment with RUXIENCE within 24 weeks after the last induction infusion with a rituximab product or based on clinical evaluation, but no sooner than 16 weeks after the last induction infusion with a rituximab product.
- •
- If induction treatment of active disease was with other standard of care immunosuppressants, initiate RUXIENCE follow up treatment within the 4 week period that follows achievement of disease control.
2.7 Recommended Dose for Premedication and Prophylactic Medications
Premedicate with acetaminophen and an antihistamine before each infusion of RUXIENCE. For adult patients administered RUXIENCE according to the 90-minute infusion rate, the glucocorticoid component of their chemotherapy regimen should be administered prior to infusion [see Clinical Studies (14.4)].
For RA, GPA and MPA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion.
Provide prophylaxis treatment for Pneumocystis jirovecii pneumonia (PCP) and herpes virus infections for patients with CLL during treatment and for up to 12 months following treatment as appropriate [see Warnings and Precautions (5.6)].
PCP prophylaxis is also recommended for patients with GPA and MPA during treatment and for at least 6 months following the last RUXIENCE infusion.
Close2.8 Administration and Storage
Use appropriate aseptic technique. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. RUXIENCE should be a clear to slightly opalescent, colorless to pale brownish-yellow liquid. Do not use vial if particulates or discoloration is present.
Administration
Use a sterile needle and syringe to prepare RUXIENCE. Withdraw the necessary amount of RUXIENCE and dilute to a final concentration of 1 mg/mL to 4 mg/mL in an infusion bag containing either 0.9% Sodium Chloride Injection or 5% Dextrose Injection. Gently invert the bag to mix the solution. Do not mix or dilute with other drugs. Discard any unused portion left in the vial.
Storage
If not used immediately, store diluted RUXIENCE solutions as shown in the table below.
Diluted RUXIENCE Solution Storage Conditions
Diluent Used to Prepare Solution for Infusion
Diluted RUXIENCE Solution Storage Conditions
0.9% Sodium Chloride Injection, USP
Store RUXIENCE solution diluted in 0.9% Sodium Chloride Injection, USP refrigerated at 2°C to 8°C (36°F to 46°F) for up to 16 days after preparation.
5% Dextrose Injection, USP
Store RUXIENCE solution diluted in 5% Dextrose Injection, USP refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours after preparation.
No incompatibilities between RUXIENCE and polyvinylchloride bags have been observed.
-
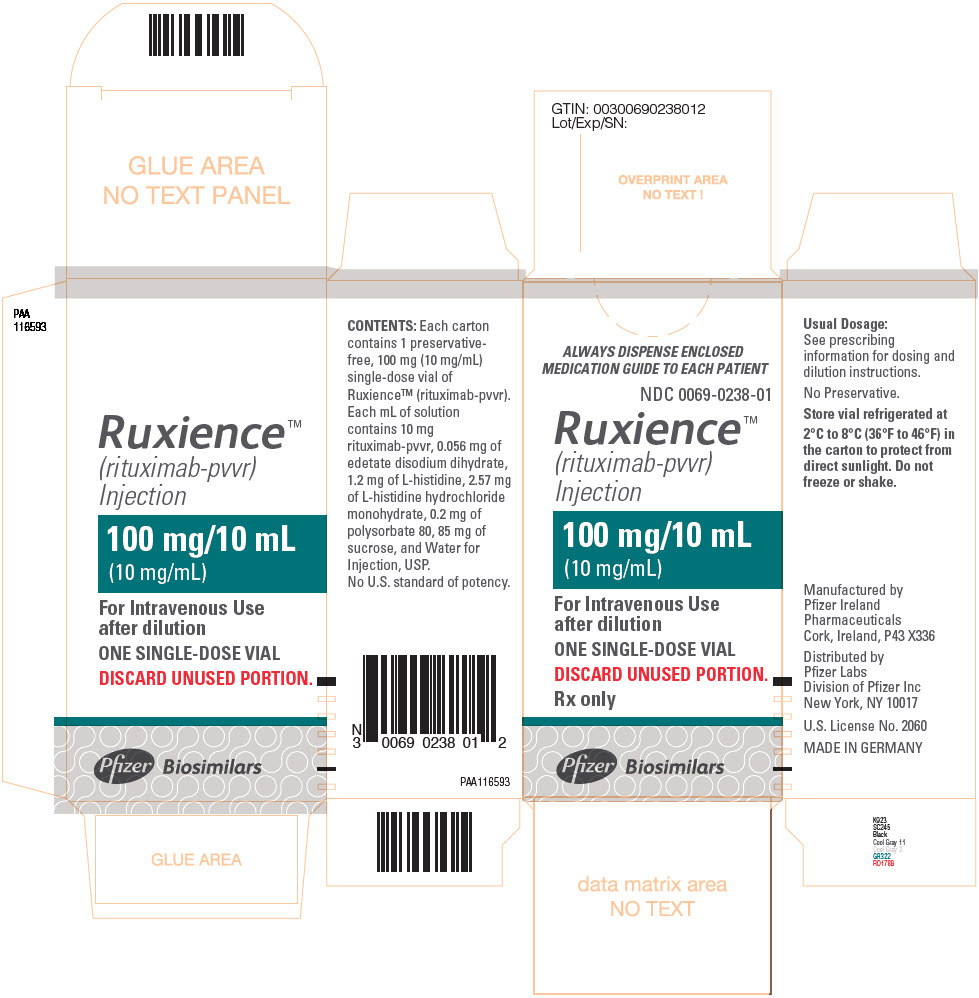
3 DOSAGE FORMS AND STRENGTHSInjection: RUXIENCE is a clear to slightly opalescent, colorless to pale brownish-yellow solution for intravenous infusion: • 100 mg/10 mL (10 mg/mL) in a single-dose vial - • 500 mg/50 mL (10 ...
Injection: RUXIENCE is a clear to slightly opalescent, colorless to pale brownish-yellow solution for intravenous infusion:
- •
- 100 mg/10 mL (10 mg/mL) in a single-dose vial
- •
- 500 mg/50 mL (10 mg/mL) in a single-dose vial
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion-Related Reactions - Rituximab products can cause severe, including fatal, infusion-related reactions. Severe reactions typically occurred during the first infusion with time to onset ...
5.1 Infusion-Related Reactions
Rituximab products can cause severe, including fatal, infusion-related reactions. Severe reactions typically occurred during the first infusion with time to onset of 30 to 120 minutes. Rituximab product-induced infusion-related reactions and sequelae include urticaria, hypotension, angioedema, hypoxia, bronchospasm, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, cardiogenic shock, anaphylactoid events, or death.
Premedicate patients with an antihistamine and acetaminophen prior to dosing. For RA, GPA and MPA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion. Institute medical management (e.g., glucocorticoids, epinephrine, bronchodilators, or oxygen) for infusion-related reactions as needed. Depending on the severity of the infusion-related reaction and the required interventions, temporarily or permanently discontinue RUXIENCE. Resume infusion at a minimum 50% reduction in rate after symptoms have resolved. Closely monitor the following patients: those with pre-existing cardiac or pulmonary conditions, those who experienced prior cardiopulmonary adverse reactions, and those with high numbers of circulating malignant cells (greater than or equal to 25,000/mm3) [see Warnings and Precautions (5.7), Adverse Reactions (6.1)].
5.2 Severe Mucocutaneous Reactions
Mucocutaneous reactions, some with fatal outcome, can occur in patients treated with rituximab products. These reactions include paraneoplastic pemphigus, Stevens-Johnson syndrome, lichenoid dermatitis, vesiculobullous dermatitis, and toxic epidermal necrolysis. The onset of these reactions has been variable and includes reports with onset on the first day of rituximab exposure. Discontinue RUXIENCE in patients who experience a severe mucocutaneous reaction. The safety of re-administration of rituximab products to patients with severe mucocutaneous reactions has not been determined.
5.3 Hepatitis B Virus (HBV) Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs classified as CD20-directed cytolytic antibodies, including rituximab products. Cases have been reported in patients who are hepatitis B surface antigen (HBsAg) positive and also in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation also has occurred in patients who appear to have resolved hepatitis B infection (i.e., HBsAg negative, anti-HBc positive and hepatitis B surface antibody [anti-HBs] positive).
HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA levels or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, i.e., increase in transaminase levels. In severe cases increase in bilirubin levels, liver failure, and death can occur.
Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with RUXIENCE. For patients who show evidence of prior hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult with physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy before and/or during RUXIENCE treatment.
Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following RUXIENCE therapy. HBV reactivation has been reported up to 24 months following completion of rituximab therapy.
In patients who develop reactivation of HBV while on RUXIENCE, immediately discontinue RUXIENCE and any concomitant chemotherapy, and institute appropriate treatment. Insufficient data exist regarding the safety of resuming RUXIENCE treatment in patients who develop HBV reactivation. Resumption of RUXIENCE treatment in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing HBV.
5.4 Progressive Multifocal Leukoencephalopathy (PML)
JC virus infection resulting in PML and death can occur in rituximab product-treated patients with hematologic malignancies or with autoimmune diseases. The majority of patients with hematologic malignancies diagnosed with PML received rituximab in combination with chemotherapy or as part of a hematopoietic stem cell transplant. The patients with autoimmune diseases had prior or concurrent immunosuppressive therapy. Most cases of PML were diagnosed within 12 months of their last infusion of rituximab.
Consider the diagnosis of PML in any patient presenting with new-onset neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture.
Discontinue RUXIENCE and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
5.5 Tumor Lysis Syndrome (TLS)
Acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, or hyperphosphatemia from tumor lysis, sometimes fatal, can occur within 12–24 hours after the first infusion of rituximab products in patients with NHL. A high number of circulating malignant cells (greater than or equal to 25,000/mm3) or high tumor burden, confers a greater risk of TLS.
Administer aggressive intravenous hydration and anti-hyperuricemic therapy in patients at high risk for TLS. Correct electrolyte abnormalities, monitor renal function and fluid balance, and administer supportive care, including dialysis as indicated [see Warnings and Precautions (5.8)].
5.6 Infections
Serious, including fatal, bacterial, fungal, and new or reactivated viral infections can occur during and following the completion of rituximab product-based therapy. Infections have been reported in some patients with prolonged hypogammaglobulinemia (defined as hypogammaglobulinemia greater than 11 months after rituximab exposure). New or reactivated viral infections included cytomegalovirus, herpes simplex virus, parvovirus B19, varicella zoster virus, West Nile virus, and hepatitis B and C. Discontinue RUXIENCE for serious infections and institute appropriate anti-infective therapy [see Adverse Reactions (6.1, 6.3)]. RUXIENCE is not recommended for use in patients with severe, active infections.
5.7 Cardiovascular Adverse Reactions
Cardiac adverse reactions, including ventricular fibrillation, myocardial infarction, and cardiogenic shock may occur in patients receiving rituximab products. Discontinue infusions for serious or life-threatening cardiac arrhythmias. Perform cardiac monitoring during and after all infusions of RUXIENCE for patients who develop clinically significant arrhythmias, or who have a history of arrhythmia or angina [see Adverse Reactions (6.1)].
5.8 Renal Toxicity
Severe, including fatal, renal toxicity can occur after rituximab product administration in patients with NHL. Renal toxicity has occurred in patients who experience tumor lysis syndrome and in patients with NHL administered concomitant cisplatin therapy during clinical trials. The combination of cisplatin and RUXIENCE is not an approved treatment regimen. Monitor closely for signs of renal failure and discontinue RUXIENCE in patients with a rising serum creatinine or oliguria [see Warnings and Precautions (5.5)].
5.9 Bowel Obstruction and Perforation
Abdominal pain, bowel obstruction and perforation, in some cases leading to death, can occur in patients receiving rituximab products in combination with chemotherapy. In postmarketing reports, the mean time to documented gastrointestinal perforation was 6 (range 1–77) days in patients with NHL. Evaluate if symptoms of obstruction such as abdominal pain or repeated vomiting occur.
5.10 Immunization
The safety of immunization with live viral vaccines following rituximab product therapy has not been studied and vaccination with live virus vaccines is not recommended before or during treatment.
For patients treated with RUXIENCE, physicians should review the patient's vaccination status and patients should, if possible, be brought up-to-date with all immunizations in agreement with current immunization guidelines prior to initiating RUXIENCE and administer non live vaccines at least 4 weeks prior to a course of RUXIENCE.
The effect of rituximab on immune responses was assessed in a randomized, controlled study in patients with RA treated with rituximab and methotrexate (MTX) compared to patients treated with MTX alone.
A response to pneumococcal vaccination (a T-cell independent antigen) as measured by an increase in antibody titers to at least 6 of 12 serotypes was lower in patients treated with rituximab plus MTX as compared to patients treated with MTX alone (19% vs. 61%). A lower proportion of patients in the rituximab plus MTX group developed detectable levels of anti-keyhole limpet hemocyanin antibodies (a novel protein antigen) after vaccination compared to patients on MTX alone (47% vs. 93%).
A positive response to tetanus toxoid vaccine (a T-cell dependent antigen with existing immunity) was similar in patients treated with rituximab plus MTX compared to patients on MTX alone (39% vs. 42%). The proportion of patients maintaining a positive Candida skin test (to evaluate delayed type hypersensitivity) was also similar (77% of patients on rituximab plus MTX vs. 70% of patients on MTX alone).
Most patients in the rituximab-treated group had B-cell counts below the lower limit of normal at the time of immunization. The clinical implications of these findings are not known.
5.11 Embryo-Fetal Toxicity
Based on human data, rituximab products can cause fetal harm due to B-cell lymphocytopenia in infants exposed in-utero. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception while receiving RUXIENCE and for 12 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.12 Concomitant Use with Other Biologic Agents and DMARDS other than Methotrexate in RA, GPA and MPA
Limited data are available on the safety of the use of biologic agents or disease modifying anti-rheumatic drugs (DMARDs) other than methotrexate in RA patients exhibiting peripheral B-cell depletion following treatment with rituximab. Observe patients closely for signs of infection if biologic agents and/or DMARDs are used concomitantly. Use of concomitant immunosuppressants other than corticosteroids has not been studied in GPA or MPA patients exhibiting peripheral B-cell depletion following treatment with rituximab products.
Close5.13 Use in RA Patients Who Have Not Had Prior Inadequate Response to Tumor Necrosis Factor (TNF) Antagonists
While the efficacy of rituximab was supported in four controlled trials in patients with RA with prior inadequate responses to non-biologic DMARDs, and in a controlled trial in MTX-naïve patients, a favorable risk-benefit relationship has not been established in these populations. The use of RUXIENCE in patients with RA who have not had prior inadequate response to one or more TNF antagonists is not recommended [see Clinical Studies (14.6)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: • Infusion-related reactions [see Warnings and Precautions (5.1)] ...
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Infusion-related reactions [see Warnings and Precautions (5.1)]
- •
- Severe mucocutaneous reactions [see Warnings and Precautions (5.2)]
- •
- Hepatitis B reactivation with fulminant hepatitis [see Warnings and Precautions (5.3)]
- •
- Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.4)]
- •
- Tumor lysis syndrome [see Warnings and Precautions (5.5)]
- •
- Infections [see Warnings and Precautions (5.6)]
- •
- Cardiovascular adverse reactions [see Warnings and Precautions (5.7)]
- •
- Renal toxicity [see Warnings and Precautions (5.8)]
- •
- Bowel obstruction and perforation [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
B-Cell Malignancies
The data described below reflect exposure to rituximab in 3,092 patients, with exposures ranging from a single infusion up to 2 years. Rituximab was studied in both single-arm and controlled trials (n=356 and n=2,427). The population included 1,180 patients with low grade or follicular lymphoma, 927 patients with DLBCL, 676 patients with CLL, and 309 patients with another indication. Most NHL patients received rituximab as an infusion of 375 mg/m2 per infusion, given as a single agent weekly for up to 8 doses, in combination with chemotherapy for up to 8 doses, or following chemotherapy for up to 16 doses. CLL patients received rituximab 375 mg/m2 as an initial infusion followed by 500 mg/m2 for up to 5 doses, in combination with fludarabine and cyclophosphamide. Seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of rituximab-based therapy.
The most common adverse reactions of rituximab (incidence greater than or equal to 25%) observed in clinical trials of patients with NHL were infusion-related reactions, fever, lymphopenia, chills, infection, and asthenia.
The most common adverse reactions of rituximab (incidence greater than or equal to 25%) observed in clinical trials of patients with CLL were: infusion-related reactions and neutropenia.
Infusion-Related Reactions
In the majority of patients with NHL, infusion-related reactions consisting of fever, chills/rigors, nausea, pruritus, angioedema, hypotension, headache, bronchospasm, urticaria, rash, vomiting, myalgia, dizziness, or hypertension occurred during the first rituximab infusion. Infusion-related reactions typically occurred within 30 to 120 minutes of beginning the first infusion and resolved with slowing or interruption of the rituximab infusion and with supportive care (diphenhydramine, acetaminophen, and intravenous saline). The incidence of infusion-related reactions was highest during the first infusion (77%) and decreased with each subsequent infusion [see Warnings and Precautions (5.1)]. In adult patients with previously untreated follicular NHL or previously untreated DLBCL, who did not experience a Grade 3 or 4 infusion-related reaction in Cycle 1 and received a 90-minute infusion of rituximab at Cycle 2, the incidence of Grade 3–4 infusion-related reactions on the day of, or day after the infusion was 1.1% (95% CI [0.3%, 2.8%]). For Cycles 2–8, the incidence of Grade 3–4 infusion-related reactions on the day of or day after the 90-minute infusion, was 2.8% (95% CI [1.3%, 5.0%]) [see Warnings and Precautions (5.1), Clinical Studies (14.4)].
Infections
Serious infections (NCI CTCAE Grade 3 or 4), including sepsis, occurred in less than 5% of patients with NHL in the single-arm studies. The overall incidence of infections was 31% (bacterial 19%, viral 10%, unknown 6%, and fungal 1%) [see Warnings and Precautions (5.6)].
In randomized, controlled studies where rituximab was administered following chemotherapy for the treatment of follicular or low-grade NHL, the rate of infection was higher among patients who received rituximab. In diffuse large B-cell lymphoma patients, viral infections occurred more frequently in those who received rituximab.
Cytopenias and Hypogammaglobulinemia
In patients with NHL receiving rituximab monotherapy, NCI-CTC Grade 3 and 4 cytopenias were reported in 48% of patients. These included lymphopenia (40%), neutropenia (6%), leukopenia (4%), anemia (3%), and thrombocytopenia (2%). The median duration of lymphopenia was 14 days (range, 1–588 days) and of neutropenia was 13 days (range, 2–116 days). A single occurrence of transient aplastic anemia (pure red cell aplasia) and two occurrences of hemolytic anemia following rituximab therapy occurred during the single-arm studies.
In studies of monotherapy, rituximab-induced B-cell depletion occurred in 70% to 80% of patients with NHL. Decreased IgM and IgG serum levels occurred in 14% of these patients.
In CLL trials, the frequency of prolonged neutropenia and late-onset neutropenia was higher in patients treated with rituximab in combination with fludarabine and cyclophosphamide (R-FC) compared to patients treated with FC. Prolonged neutropenia is defined as Grade 3–4 neutropenia that has not resolved between 24 and 42 days after the last dose of study treatment. Late-onset neutropenia is defined as Grade 3–4 neutropenia starting at least 42 days after the last treatment dose.
In patients with previously untreated CLL, the frequency of prolonged neutropenia was 8.5% for patients who received R-FC (n=402) and 5.8% for patients who received FC (n=398). In patients who did not have prolonged neutropenia, the frequency of late-onset neutropenia was 14.8% of 209 patients who received R-FC and 4.3% of 230 patients who received FC.
For patients with previously treated CLL, the frequency of prolonged neutropenia was 24.8% for patients who received R-FC (n=274) and 19.1% for patients who received FC (n=274). In patients who did not have prolonged neutropenia, the frequency of late-onset neutropenia was 38.7% in 160 patients who received R-FC and 13.6% of 147 patients who received FC.
Relapsed or Refractory, Low-Grade NHL
Adverse reactions presented in Table 1 occurred in 356 patients with relapsed or refractory, low-grade or follicular, CD20-positive, B-cell NHL treated in single-arm studies of rituximab administered as a single agent [see Clinical Studies (14.1)]. Most patients received rituximab 375 mg/m2 weekly for 4 doses.
Table 1. Incidence of Adverse Reactions in Greater than or Equal to 5% of Patients with Relapsed or Refractory, Low-Grade or Follicular NHL, Receiving Single-agent Rituximab (N=356)*,† All Grades (%) Grade 3 and 4 (%) Any Adverse Reactions
99
57
Body as a Whole
86
10
Fever
53
1
Chills
33
3
Infection
31
4
Asthenia
26
1
Headache
19
1
Abdominal Pain
14
1
Pain
12
1
Back Pain
10
1
Throat Irritation
9
0
Flushing
5
0
Heme and Lymphatic System
67
48
Lymphopenia
48
40
Leukopenia
14
4
Neutropenia
14
6
Thrombocytopenia
12
2
Anemia
8
3
Skin and Appendages
44
2
Night Sweats
15
1
Rash
15
1
Pruritus
14
1
Urticaria
8
1
Respiratory System
38
4
Increased Cough
13
1
Rhinitis
12
1
Bronchospasm
8
1
Dyspnea
7
1
Sinusitis
6
0
Metabolic and Nutritional Disorders
38
3
Angioedema
11
1
Hyperglycemia
9
1
Peripheral Edema
8
0
LDH Increase
7
0
Digestive System
37
2
Nausea
23
1
Diarrhea
10
1
Vomiting
10
1
Nervous System
32
1
Dizziness
10
1
Anxiety
5
1
Musculoskeletal System
26
3
Myalgia
10
1
Arthralgia
10
1
Cardiovascular System
25
3
Hypotension
10
1
Hypertension
6
1
In these single-arm rituximab studies, bronchiolitis obliterans occurred during and up to 6 months after rituximab infusion.
Previously Untreated, Low-Grade or Follicular, NHL
In NHL Study 4, patients in the R-CVP arm experienced a higher incidence of infusional toxicity and neutropenia compared to patients in the CVP arm. The following adverse reactions occurred more frequently (greater than or equal to 5%) in patients receiving R-CVP compared to CVP alone: rash (17% vs. 5%), cough (15% vs. 6%), flushing (14% vs. 3%), rigors (10% vs. 2%), pruritus (10% vs. 1%), neutropenia (8% vs. 3%), and chest tightness (7% vs. 1%) [see Clinical Studies (14.2)].
In NHL Study 5, detailed safety data collection was limited to serious adverse reactions, Grade greater than or equal to 2 infections, and Grade greater than or equal to 3 adverse reactions. In patients receiving rituximab as single-agent maintenance therapy following rituximab plus chemotherapy, infections were reported more frequently compared to the observation arm (37% vs. 22%). Grade 3-4 adverse reactions occurring at a higher incidence (greater than or equal to 2%) in the rituximab group were infections (4% vs. 1%) and neutropenia (4% vs. less than 1%).
In NHL Study 6, the following adverse reactions were reported more frequently (greater than or equal to 5%) in patients receiving rituximab following CVP compared to patients who received no further therapy: fatigue (39% vs. 14%), anemia (35% vs. 20%), peripheral sensory neuropathy (30% vs. 18%), infections (19% vs. 9%), pulmonary toxicity (18% vs. 10%), hepato-biliary toxicity (17% vs. 7%), rash and/or pruritus (17% vs. 5%), arthralgia (12% vs. 3%), and weight gain (11% vs. 4%). Neutropenia was the only Grade 3 or 4 adverse reaction that occurred more frequently (greater than or equal to 2%) in the rituximab arm compared with those who received no further therapy (4% vs. 1%) [see Clinical Studies (14.3)].
DLBCL
In NHL Studies 7 (NCT00003150) and 8, [see Clinical Studies (14.3)], the following adverse reactions, regardless of severity, were reported more frequently (greater than or equal to 5%) in patients age greater than or equal to 60 years receiving R-CHOP as compared to CHOP alone: pyrexia (56% vs. 46%), lung disorder (31% vs. 24%), cardiac disorder (29% vs. 21%), and chills (13% vs. 4%). Detailed safety data collection in these studies was primarily limited to Grade 3 and 4 adverse reactions and serious adverse reactions.
In NHL Study 8, a review of cardiac toxicity determined that supraventricular arrhythmias or tachycardia accounted for most of the difference in cardiac disorders (4.5% for R-CHOP vs. 1.0% for CHOP).
The following Grade 3 or 4 adverse reactions occurred more frequently among patients in the R-CHOP arm compared with those in the CHOP arm: thrombocytopenia (9% vs. 7%) and lung disorder (6% vs. 3%). Other Grade 3 or 4 adverse reactions occurring more frequently among patients receiving R-CHOP were viral infection (NHL Study 8), neutropenia (NHL Studies 8 and 9 (NCT00064116)), and anemia (NHL Study 9).
CLL
The data below reflect exposure to rituximab in combination with fludarabine and cyclophosphamide in 676 patients with CLL in CLL Study 1 (NCT00281918) or CLL Study 2 (NCT00090051) [see Clinical Studies (14.5)]. The age range was 30–83 years and 71% were men. Detailed safety data collection in CLL Study 1 was limited to Grade 3 and 4 adverse reactions and serious adverse reactions.
Infusion-related adverse reactions were defined by any of the following adverse events occurring during or within 24 hours of the start of infusion: nausea, pyrexia, chills, hypotension, vomiting, and dyspnea.
In CLL Study 1, the following Grade 3 and 4 adverse reactions occurred more frequently in R-FC-treated patients compared to FC-treated patients: infusion-related reactions (9% in R-FC arm), neutropenia (30% vs. 19%), febrile neutropenia (9% vs. 6%), leukopenia (23% vs. 12%), and pancytopenia (3% vs. 1%).
In CLL Study 2, the following Grade 3 or 4 adverse reactions occurred more frequently in R-FC-treated patients compared to FC-treated patients: infusion-related reactions (7% in R-FC arm), neutropenia (49% vs. 44%), febrile neutropenia (15% vs. 12%), thrombocytopenia (11% vs. 9%), hypotension (2% vs. 0%), and hepatitis B (2% vs. less than 1%). Fifty-nine percent of R-FC-treated patients experienced an infusion-related reaction of any severity.
Rheumatoid Arthritis
The data presented below reflect the experience in 2,578 RA patients treated with rituximab in controlled and long-term studies11 with a total exposure of 5,014 patient years.
Among all exposed patients, adverse reactions reported in greater than 10% of patients include infusion-related reactions, upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis.
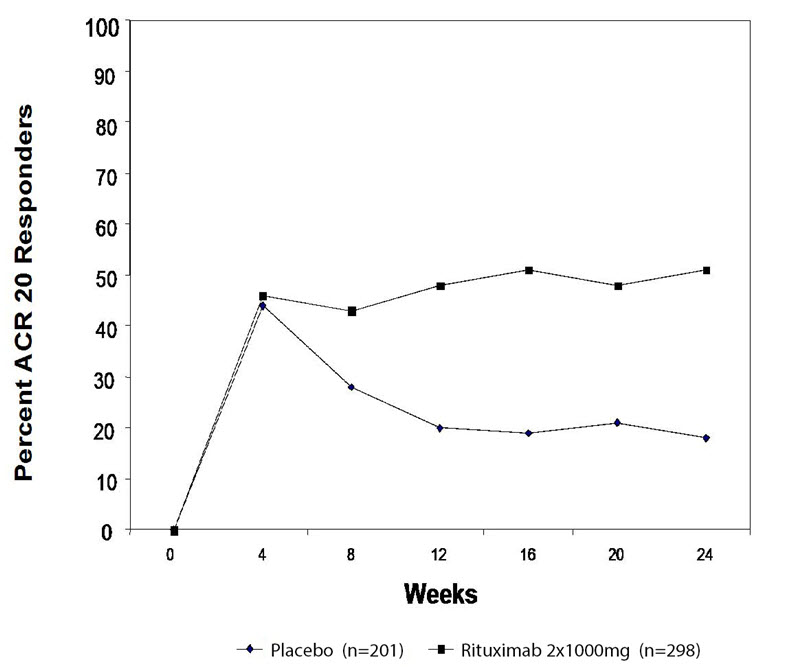
In placebo-controlled studies, patients received 2 × 500 mg or 2 × 1,000 mg intravenous infusions of rituximab or placebo, in combination with methotrexate, during a 24-week period. From these studies, 938 patients treated with rituximab (2 × 1,000 mg) or placebo have been pooled (see Table 2). Adverse reactions reported in greater than or equal to 5% of patients were hypertension, nausea, upper respiratory tract infection, arthralgia, pyrexia and pruritus (see Table 2). The rates and types of adverse reactions in patients who received rituximab 2 × 500 mg were similar to those observed in patients who received rituximab 2 × 1,000 mg.
Table 2*Incidence of All Adverse Reactions† Occurring in Greater than or Equal to 2% and at Least 1% Greater than Placebo Among Rheumatoid Arthritis Patients in Clinical Studies Up to Week 24 (Pooled) Adverse Reaction Placebo + MTX
N=398
n (%)Rituximab + MTX
N=540
n (%)Hypertension
21 (5)
43 (8)
Nausea
19 (5)
41 (8)
Upper Respiratory Tract Infection
23 (6)
37 (7)
Arthralgia
14 (4)
31 (6)
Pyrexia
8 (2)
27 (5)
Pruritus
5 (1)
26 (5)
Chills
9 (2)
16 (3)
Dyspepsia
3 (<1)
16 (3)
Rhinitis
6 (2)
14 (3)
Paresthesia
3 (<1)
12 (2)
Urticaria
3 (<1)
12 (2)
Abdominal Pain Upper
4 (1)
11 (2)
Throat Irritation
0 (0)
11 (2)
Anxiety
5 (1)
9 (2)
Migraine
2 (<1)
9 (2)
Asthenia
1 (<1)
9 (2)
- 1
- 1 Pooled studies: NCT00074438, NCT00422383, NCT00468546, NCT00299130, NCT00282308, NCT00266227, NCT02693210, NCT02093026 and NCT02097745.
Infusion-Related Reactions
In the rituximab RA pooled placebo-controlled studies, 32% of rituximab-treated patients experienced an adverse reaction during or within 24 hours following their first infusion, compared to 23% of placebo-treated patients receiving their first infusion. The incidence of adverse reactions during the 24-hour period following the second infusion, rituximab or placebo, decreased to 11% and 13%, respectively. Acute infusion-related reactions (manifested by fever, chills, rigors, pruritus, urticaria/rash, angioedema, sneezing, throat irritation, cough, and/or bronchospasm, with or without associated hypotension or hypertension) were experienced by 27% of rituximab-treated patients following their first infusion, compared to 19% of placebo-treated patients receiving their first placebo infusion. The incidence of these acute infusion-related reactions following the second infusion of rituximab or placebo decreased to 9% and 11%, respectively. Serious acute infusion-related reactions were experienced by less than 1% of patients in either treatment group. Acute infusion-related reactions required dose modification (stopping, slowing, or interruption of the infusion) in 10% and 2% of patients receiving rituximab or placebo, respectively, after the first course. The proportion of patients experiencing acute infusion-related reactions decreased with subsequent courses of rituximab. The administration of intravenous glucocorticoids prior to rituximab infusions reduced the incidence and severity of such reactions, however, there was no clear benefit from the administration of oral glucocorticoids for the prevention of acute infusion-related reactions. Patients in clinical studies also received antihistamines and acetaminophen prior to rituximab infusions.
Infections
In the pooled, placebo-controlled studies, 39% of patients in the rituximab group experienced an infection of any type compared to 34% of patients in the placebo group. The most common infections were nasopharyngitis, upper respiratory tract infections, urinary tract infections, bronchitis, and sinusitis.
The incidence of serious infections was 2% in the rituximab-treated patients and 1% in the placebo group.
In the experience with rituximab in 2,578 RA patients, the rate of serious infections was 4.31 per 100 patient years. The most common serious infections (greater than or equal to 0.5%) were pneumonia or lower respiratory tract infections, cellulitis and urinary tract infections. Fatal serious infections included pneumonia, sepsis and colitis. Rates of serious infection remained stable in patients receiving subsequent courses. In 185 rituximab-treated RA patients with active disease, subsequent treatment with a biologic DMARD, the majority of which were TNF antagonists, did not appear to increase the rate of serious infection. Thirteen serious infections were observed in 186.1 patient years (6.99 per 100 patient years) prior to exposure and 10 were observed in 182.3 patient years (5.49 per 100 patient years) after exposure.
Cardiovascular Adverse Reactions
In the pooled, placebo-controlled studies, the proportion of patients with serious cardiovascular reactions was 1.7% and 1.3% in the rituximab and placebo treatment groups, respectively. Three cardiovascular deaths occurred during the double-blind period of the RA studies including all rituximab regimens (3/769=0.4%) as compared to none in the placebo treatment group (0/389).
In the experience with rituximab in 2,578 RA patients, the rate of serious cardiac reactions was 1.93 per 100 patient years. The rate of myocardial infarction (MI) was 0.56 per 100 patient years (28 events in 26 patients), which is consistent with MI rates in the general RA population. These rates did not increase over three courses of rituximab.
Since patients with RA are at increased risk for cardiovascular events compared with the general population, patients with RA should be monitored throughout the infusion and RUXIENCE should be discontinued in the event of a serious or life-threatening cardiac event.
Hypophosphatemia and Hyperuricemia
In the pooled, placebo-controlled studies, newly-occurring hypophosphatemia (less than 2.0 mg/dL) was observed in 12% (67/540) of patients on rituximab versus 10% (39/398) of patients on placebo. Hypophosphatemia was more common in patients who received corticosteroids. Newly-occurring hyperuricemia (greater than 10 mg/dL) was observed in 1.5% (8/540) of patients on rituximab versus 0.3% (1/398) of patients on placebo.
In the experience with rituximab in RA patients, newly-occurring hypophosphatemia was observed in 21% (528/2570) of patients and newly-occurring hyperuricemia was observed in 2% (56/2570) of patients. The majority of the observed hypophosphatemia occurred at the time of the infusions and was transient.
Retreatment in Patients with RA
In the experience with rituximab in RA patients, 2,578 patients have been exposed to rituximab and have received up to 10 courses of rituximab in RA clinical trials, with 1,890, 1,043, and 425 patients having received at least two, three, and four courses, respectively. Most of the patients who received additional courses did so 24 weeks or more after the previous course and none were retreated sooner than 16 weeks. The rates and types of adverse reactions reported for subsequent courses of rituximab were similar to rates and types seen for a single course of rituximab.
In RA Study 2, where all patients initially received rituximab, the safety profile of patients who were retreated with rituximab was similar to those who were retreated with placebo [see Clinical Studies (14.6), Dosage and Administration (2.5)].
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Induction Treatment of Adult Patients with Active GPA/MPA (GPA/MPA Study 1)
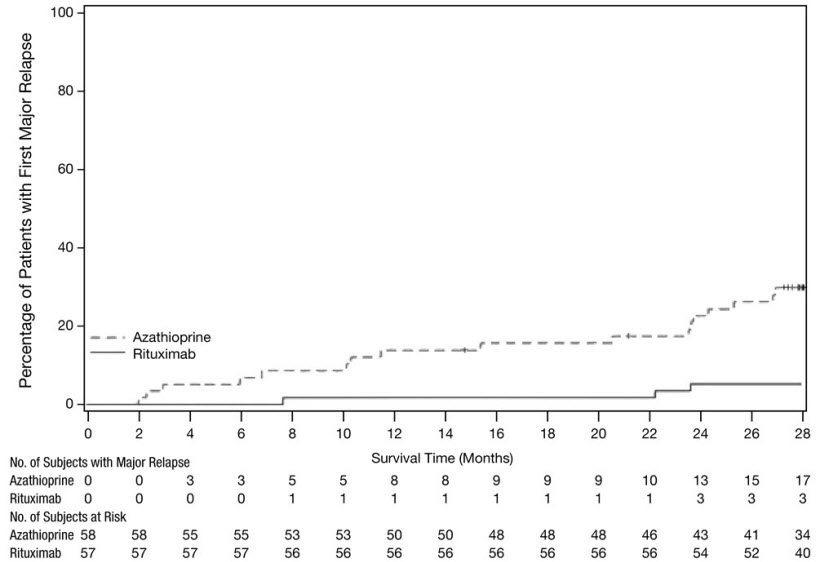
The data presented below from GPA/MPA Study 1 (NCT00104299) reflect the experience in 197 adult patients with active GPA and MPA treated with rituximab or cyclophosphamide in a single controlled study, which was conducted in two phases: a 6 month randomized, double-blind, double-dummy, active-controlled remission induction phase and an additional 12 month remission maintenance phase [see Clinical Studies (14.7)]. In the 6-month remission induction phase, 197 patients with GPA and MPA were randomized to either rituximab 375 mg/m2 once weekly for 4 weeks plus glucocorticoids, or oral cyclophosphamide 2 mg/kg daily (adjusted for renal function, white blood cell count, and other factors) plus glucocorticoids to induce remission. Once remission was achieved or at the end of the 6 month remission induction period, the cyclophosphamide group received azathioprine to maintain remission. The rituximab group did not receive additional therapy to maintain remission. The primary analysis was at the end of the 6 month remission induction period and the safety results for this period are described below.
Adverse reactions presented below in Table 3 were adverse events which occurred at a rate of greater than or equal to 10% in the rituximab group. This table reflects experience in 99 GPA and MPA patients treated with rituximab, with a total of 47.6 patient years of observation and 98 GPA and MPA patients treated with cyclophosphamide, with a total of 47.0 patient years of observation. Infection was the most common category of adverse events reported (47–62%) and is discussed below.
Table 3 Incidence of All Adverse Reactions Occurring in Greater than or Equal to 10% of Rituximab-treated Patients with active GPA and MPA in the GPA/MPA Study 1 Up to Month 6* Adverse Reaction Rituximab
N=99
n (%)Cyclophosphamide
N=98
n (%)- *
- The study design allowed for crossover or treatment by best medical judgment, and 13 patients in each treatment group received a second therapy during the 6 month study period.
Nausea
18 (18%)
20 (20%)
Diarrhea
17 (17%)
12 (12%)
Headache
17 (17%)
19 (19%)
Muscle Spasms
17 (17%)
15 (15%)
Anemia
16 (16%)
20 (20%)
Peripheral Edema
16 (16%)
6 (6%)
Insomnia
14 (14%)
12 (12%)
Arthralgia
13 (13%)
9 (9%)
Cough
13 (13%)
11 (11%)
Fatigue
13 (13%)
21 (21%)
Increased ALT
13 (13%)
15 (15%)
Hypertension
12 (12%)
5 (5%)
Epistaxis
11 (11%)
6 (6%)
Dyspnea
10 (10%)
11 (11%)
Leukopenia
10 (10%)
26 (27%)
Rash
10 (10%)
17 (17%)
Infusion-Related Reactions
Infusion-related reactions in GPA/MPA Study 1 were defined as any adverse event occurring within 24 hours of an infusion and considered to be infusion-related by investigators. Among the 99 patients treated with rituximab, 12% experienced at least one infusion-related reaction, compared with 11% of the 98 patients in the cyclophosphamide group. Infusion-related reactions included cytokine release syndrome, flushing, throat irritation, and tremor. In the rituximab group, the proportion of patients experiencing an infusion-related reaction was 12%, 5%, 4%, and 1% following the first, second, third, and fourth infusions, respectively. Patients were pre-medicated with antihistamine and acetaminophen before each rituximab infusion and were on background oral corticosteroids which may have mitigated or masked an infusion-related reaction; however, there is insufficient evidence to determine whether premedication diminishes the frequency or severity of infusion-related reactions.
Infections
In GPA/MPA Study 1, 62% (61/99) of patients in the rituximab group experienced an infection of any type compared to 47% (46/98) patients in the cyclophosphamide group by Month 6. The most common infections in the rituximab group were upper respiratory tract infections, urinary tract infections, and herpes zoster.
The incidence of serious infections was 11% in the rituximab-treated patients and 10% in the cyclophosphamide treated patients, with rates of approximately 25 and 28 per 100 patient years, respectively. The most common serious infection was pneumonia.
Hypogammaglobulinemia
Hypogammaglobulinemia (IgA, IgG, or IgM below the lower limit of normal) has been observed in patients with GPA and MPA treated with rituximab in GPA/MPA Study 1. At 6 months, in the rituximab group, 27%, 58% and 51% of patients with normal immunoglobulin levels at baseline, had low IgA, IgG and IgM levels, respectively compared to 25%, 50%, and 46% in the cyclophosphamide group.
Follow up Treatment of Adult Patients with GPA/MPA who have Achieved Disease Control with Induction Treatment (GPA/MPA Study 2)
In GPA/MPA Study 2 (NCT00748644), an open-label, controlled, clinical study [see Clinical Studies (14.7)], evaluating the efficacy and safety of non-U.S.-licensed rituximab versus azathioprine as follow up treatment in adult patients with GPA, MPA or renal-limited ANCA-associated vasculitis who had achieved disease control after induction treatment with cyclophosphamide, a total of 57 GPA and MPA patients in disease remission received follow up treatment with two 500 mg intravenous infusions of non-U.S.-licensed rituximab, separated by two weeks on Day 1 and Day 15, followed by a 500 mg intravenous infusion every 6 months for 18 months.
The safety profile was consistent with the safety profile for rituximab in RA, GPA, and MPA.
Infusion-Related Reactions
In GPA/MPA Study 2, 7/57 (12%) patients in the non-U.S.-licensed rituximab arm reported infusion-related reactions. The incidence of IRR symptoms was highest during or after the first infusion (9%) and decreased with subsequent infusions (less than 4%). One patient had two serious IRRs, two IRRs led to a dose modification, and no IRRs were severe, fatal, or led to withdrawal from the study.
Infections
In GPA/MPA Study 2, 30/57 (53%) patients in the non-U.S.-licensed rituximab arm and 33/58 (57%) in the azathioprine arm reported infections. The incidence of all grade infections was similar between the arms. The incidence of serious infections was similar in both arms (12%). The most commonly reported serious infection in the group was mild or moderate bronchitis.
Long-term, Observational Study with Rituximab in Patients with GPA/MPA (GPA/MPA Study 3)
In a long-term observational safety study (NCT01613599), 97 patients with GPA or MPA received treatment with rituximab (mean of 8 infusions [range 1–28]) for up to 4 years, according to physician standard practice and discretion. Majority of patients received doses ranging from 500 mg to 1,000 mg, approximately every 6 months. The safety profile was consistent with the safety profile for rituximab in RA, GPA and MPA.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other rituximab products may be misleading.
Using an ELISA assay, anti-rituximab antibody was detected in 4 of 356 (1.1%) patients with low-grade or follicular NHL receiving single-agent rituximab. Three of the four patients had an objective clinical response.
A total of 273/2578 (11%) patients with RA tested positive for anti-rituximab antibodies at any time after receiving rituximab. Anti-rituximab antibody positivity was not associated with increased rates of infusion-related reactions or other adverse events. Upon further treatment, the proportions of patients with infusion-related reactions were similar between anti-rituximab antibody positive and negative patients, and most reactions were mild to moderate. Four anti-rituximab antibody positive patients had serious infusion-related reactions, and the temporal relationship between anti-rituximab antibody positivity and infusion-related reaction was variable.
A total of 23/99 (23%) rituximab-treated adult patients with GPA and MPA developed anti-rituximab antibodies by 18 months in GPA/MPA Study 1. The clinical relevance of anti-rituximab antibody formation in rituximab-treated adult patients is unclear.
Close6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of rituximab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Hematologic: prolonged pancytopenia, marrow hypoplasia, Grade 3–4 prolonged or late-onset neutropenia, hyperviscosity syndrome in Waldenstrom's macroglobulinemia, prolonged hypogammaglobulinemia [see Warnings and Precautions (5.6)].
- •
- Cardiac: fatal cardiac failure.
- •
- Immune/Autoimmune Events: uveitis, optic neuritis, systemic vasculitis, pleuritis, lupus-like syndrome, serum sickness, polyarticular arthritis, and vasculitis with rash.
- •
- Infection: viral infections, including progressive multifocal leukoencephalopathy (PML), increase in fatal infections in HIV-associated lymphoma, and a reported increased incidence of Grade 3 and 4 infections [see Warnings and Precautions (5.6)].
- •
- Neoplasia: disease progression of Kaposi's sarcoma.
- •
- Skin: severe mucocutaneous reactions, pyoderma gangrenosum (including genital presentation).
- •
- Gastrointestinal: bowel obstruction and perforation.
- •
- Pulmonary: fatal bronchiolitis obliterans and fatal interstitial lung disease.
- •
- Nervous system: Posterior Reversible Encephalopathy Syndrome (PRES)/Reversible Posterior Leukoencephalopathy Syndrome (RPLS).
-
7 DRUG INTERACTIONSFormal drug interaction studies have not been performed with rituximab products. In patients with CLL, rituximab did not alter systemic exposure to fludarabine or cyclophosphamide. In clinical ...
Formal drug interaction studies have not been performed with rituximab products. In patients with CLL, rituximab did not alter systemic exposure to fludarabine or cyclophosphamide. In clinical trials of patients with RA, concomitant administration of methotrexate or cyclophosphamide did not alter the pharmacokinetics of rituximab.
Close -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on human data, rituximab products can cause adverse developmental outcomes including B-cell lymphocytopenia in infants exposed in-utero (see Clinical ...
8.1 Pregnancy
Risk Summary
Based on human data, rituximab products can cause adverse developmental outcomes including B-cell lymphocytopenia in infants exposed in-utero (see Clinical Considerations). In animal reproduction studies, intravenous administration of rituximab to pregnant cynomolgus monkeys during the period of organogenesis caused lymphoid B-cell depletion in the newborn offspring at doses resulting in 80% of the exposure (based on AUC) of those achieved following a dose of 2 grams in humans. Advise pregnant women of the risk to a fetus.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk of major birth defects and miscarriage for the indicated populations is unknown. The estimated background risk in the U.S. general population of major birth defects is 2%–4% and of miscarriage is 15%–20% of clinically recognized pregnancies.
Data
Human Data
Postmarketing data indicate that B-cell lymphocytopenia generally lasting less than six months can occur in infants exposed to rituximab in-utero. Rituximab was detected postnatally in the serum of infants exposed in-utero.
Animal Data
An embryo-fetal developmental toxicity study was performed on pregnant cynomolgus monkeys. Pregnant animals received rituximab via the intravenous route during early gestation (organogenesis period; post coitum Days 20 through 50). Rituximab was administered as loading doses on post coitum (PC) Days 20, 21, and 22, at 15, 37.5, or 75 mg/kg/day, and then weekly on PC Days 29, 36, 43, and 50, at 20, 50, or 100 mg/kg/week. The 100 mg/kg/week dose resulted in 80% of the exposure (based on AUC) of those achieved following a dose of 2 grams in humans. Rituximab crosses the monkey placenta. Exposed offspring did not exhibit any teratogenic effects but did have decreased lymphoid tissue B cells.
A subsequent pre- and postnatal reproductive toxicity study in cynomolgus monkeys was completed to assess developmental effects including the recovery of B cells and immune function in infants exposed to rituximab in-utero. Animals were treated with a loading dose of 0, 15, or 75 mg/kg every day for 3 days, followed by weekly dosing with 0, 20, or 100 mg/kg dose. Subsets of pregnant females were treated from PC Day 20 through postpartum Day 78, PC Day 76 through PC Day 134, and from PC Day 132 through delivery and postpartum Day 28. Regardless of the timing of treatment, decreased B cells and immunosuppression were noted in the offspring of rituximab-treated pregnant animals. The B-cell counts returned to normal levels, and immunologic function was restored within 6 months postpartum.
8.2 Lactation
There are limited data on the presence of rituximab in human milk, and the effect on the breastfed child, and there are no data on the effect on milk production. Rituximab is detected in the milk of lactating cynomolgus monkeys, and maternal IgG is present in human breast milk. Rituximab has also been reported to be excreted at low concentrations in human breast milk. Given that the clinical significance of this finding for children is not known, advise women not to breastfeed during treatment with RUXIENCE and for 6 months after the last dose due to the potential of serious adverse reactions in breastfed children.
8.3 Females and Males of Reproductive Potential
Rituximab products can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of RUXIENCE have not been established in pediatric patients with NHL, CLL or RA.
Rituximab was not studied in pediatric patients with polyarticular juvenile idiopathic arthritis (PJIA) due to concerns regarding the potential for prolonged immunosuppression as a result of B-cell depletion in the developing juvenile immune system.
Close8.5 Geriatric Use
Diffuse Large B-Cell NHL
Among patients with DLBCL evaluated in three randomized, active-controlled trials, 927 patients received rituximab in combination with chemotherapy. Of these, 396 (43%) were age 65 or greater and 123 (13%) were age 75 or greater. No overall differences in effectiveness were observed between these patients and younger patients. Cardiac adverse reactions, mostly supraventricular arrhythmias, occurred more frequently among elderly patients. Serious pulmonary adverse reactions were also more common among the elderly, including pneumonia and pneumonitis.
Low-Grade or Follicular Non-Hodgkin's Lymphoma
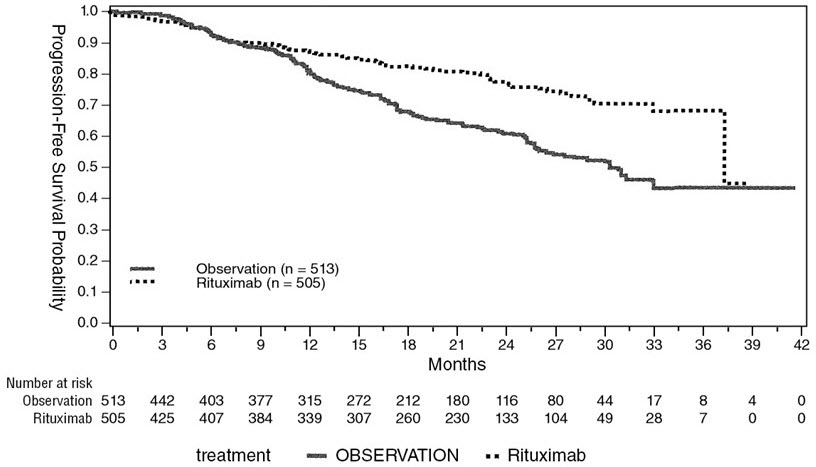
Patients with previously untreated follicular NHL evaluated in NHL Study 5 were randomized to rituximab as single-agent maintenance therapy (n=505) or observation (n=513) after achieving a response to rituximab in combination with chemotherapy. Of these, 123 (24%) patients in the rituximab arm were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients. Other clinical studies of rituximab in low-grade or follicular, CD20-positive, B-cell NHL did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
Chronic Lymphocytic Leukemia
Among patients with CLL evaluated in two randomized active-controlled trials, 243 of 676 rituximab-treated patients (36%) were 65 years of age or older; of these, 100 rituximab-treated patients (15%) were 70 years of age or older.
In exploratory analyses defined by age, there was no observed benefit from the addition of rituximab to fludarabine and cyclophosphamide among patients 70 years of age or older in CLL Study 1 or in CLL Study 2; there was also no observed benefit from the addition of rituximab to fludarabine and cyclophosphamide among patients 65 years of age or older in CLL Study 2 [see Clinical Studies (14.5)]. Patients 70 years or older received lower dose intensity of fludarabine and cyclophosphamide compared to younger patients, regardless of the addition of rituximab. In CLL Study 1, the dose intensity of rituximab was similar in older and younger patients, however in CLL Study 2 older patients received a lower dose intensity of rituximab.
The incidence of Grade 3 and 4 adverse reactions was higher among patients receiving R-FC who were 70 years or older compared to younger patients for neutropenia [44% vs. 31% (CLL Study 1); 56% vs. 39% (CLL Study 2)], febrile neutropenia [16% vs. 6% (NHL Study 10 (NCT00719472))], anemia [5% vs. 2% (CLL Study 1); 21% vs. 10% (CLL Study 2)], thrombocytopenia [19% vs. 8% (CLL Study 2)], pancytopenia [7% vs. 2% (CLL Study 1); 7% vs. 2% (CLL Study 2)], and infections [30% vs. 14% (CLL Study 2)].
Rheumatoid Arthritis
Among the 2,578 patients in global RA studies completed to date, 12% were 65–75 years old and 2% were 75 years old and older. The incidences of adverse reactions were similar between older and younger patients. The rates of serious adverse reactions, including serious infections, malignancies, and cardiovascular events were higher in older patients.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis
Of the 99 rituximab-treated GPA and MPA patients in GPA/MPA Study 1, 36 (36%) were 65 years old and over, while 8 (8%) were 75 years and over. No overall differences in efficacy were observed between patients that were 65 years old and over and younger patients. The overall incidence and rate of all serious adverse events was higher in patients 65 years old and over. The clinical study did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
In GPA/MPA Study 2, 30 (26%) of the enrolled patients were at least 65 years old, of which 12 patients were exposed to non-U.S.-licensed rituximab and 18 were exposed to azathioprine. The clinical study did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
-
11 DESCRIPTIONRituximab-pvvr is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against the CD20 antigen. Rituximab-pvvr has an approximate molecular weight of 145 ...
Rituximab-pvvr is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against the CD20 antigen. Rituximab-pvvr has an approximate molecular weight of 145 kD.
Rituximab-pvvr is produced by mammalian cell (Chinese Hamster Ovary) suspension culture in a nutrient medium.
RUXIENCE (rituximab-pvvr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale brownish-yellow solution for intravenous infusion. RUXIENCE is supplied at a concentration of 10 mg/mL in either 100 mg/10 mL or 500 mg/50 mL single-dose vials. Each mL of solution contains 10 mg rituximab-pvvr, 0.056 mg of edetate disodium dihydrate, 1.2 mg of L-histidine, 2.57 mg of L-histidine hydrochloride monohydrate, 0.2 mg of polysorbate 80, 85 mg of sucrose, and Water for Injection, USP. The pH is 5.8.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Rituximab-pvvr is a monoclonal antibody. Rituximab products target the CD20 antigen expressed on the surface of pre-B and mature B-lymphocytes. Upon binding to CD20 ...
12.1 Mechanism of Action
Rituximab-pvvr is a monoclonal antibody. Rituximab products target the CD20 antigen expressed on the surface of pre-B and mature B-lymphocytes. Upon binding to CD20, rituximab products mediate B-cell lysis. Possible mechanisms of cell lysis include complement dependent cytotoxicity (CDC) and antibody dependent cell mediated cytotoxicity (ADCC). B cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis. In this setting, B cells may be acting at multiple sites in the autoimmune/inflammatory process, including through production of rheumatoid factor (RF) and other autoantibodies, antigen presentation, T-cell activation, and/or proinflammatory cytokine production.
12.2 Pharmacodynamics
Non-Hodgkin's Lymphoma (NHL)
In NHL patients, administration of rituximab resulted in depletion of circulating and tissue-based B cells. Among 166 patients in NHL Study 1 (NCT000168740), circulating CD19-positive B cells were depleted within the first three weeks with sustained depletion for up to 6 to 9 months post treatment in 83% of patients. B-cell recovery began at approximately 6 months and median B-cell levels returned to normal by 12 months following completion of treatment.
There were sustained and statistically significant reductions in both IgM and IgG serum levels observed from 5 through 11 months following rituximab administration; 14% of patients had IgM and/or IgG serum levels below the normal range.
Rheumatoid Arthritis
In RA patients, treatment with rituximab-induced depletion of peripheral B-lymphocytes, with the majority of patients demonstrating near complete depletion (CD19 counts below the lower limit of quantification, 20 cells/µL) within 2 weeks after receiving the first dose of rituximab. The majority of patients showed peripheral B-cell depletion for at least 6 months. A small proportion of patients (~4%) had prolonged peripheral B-cell depletion lasting more than 3 years after a single course of treatment.
Total serum immunoglobulin levels, IgM, IgG, and IgA were reduced at 6 months with the greatest change observed in IgM. At Week 24 of the first course of rituximab treatment, small proportions of patients experienced decreases in IgM (10%), IgG (2.8%), and IgA (0.8%) levels below the lower limit of normal (LLN). In the experience with rituximab in RA patients during repeated rituximab treatment, 23.3%, 5.5%, and 0.5% of patients experienced decreases in IgM, IgG, and IgA concentrations below LLN at any time after receiving rituximab, respectively. The clinical consequences of decreases in immunoglobulin levels in RA patients treated with rituximab are unclear.
Treatment with rituximab in patients with RA was associated with reduction of certain biologic markers of inflammation such as interleukin-6 (IL-6), C-reactive protein (CRP), serum amyloid protein (SAA), S100 A8/S100 A9 heterodimer complex (S100 A8/9), anti-citrullinated peptide (anti-CCP), and RF.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis
In GPA and MPA patients in GPA/MPA Study 1, peripheral blood CD19 B-cells depleted to less than 10 cells/μL following the first two infusions of rituximab, and remained at that level in most (84%) patients through Month 6. By Month 12, the majority of patients (81%) showed signs of B-cell return with counts greater than 10 cells/μL. By Month 18, most patients (87%) had counts greater than 10 cells/μL.
In GPA/MPA Study 2 where patients received non-U.S.-licensed rituximab as two 500 mg intravenous infusions separated by two weeks, followed by a 500 mg intravenous infusion at Month 6, 12, and 18, 70% (30 out of 43) of the rituximab-treated patients with CD19+ peripheral B cells evaluated post-baseline had undetectable CD19+ peripheral B cells at Month 24. At Month 24, all 37 patients with evaluable baseline CD19+ peripheral B cells and Month 24 measurements had lower CD19+ B cells relative to baseline.
Close12.3 Pharmacokinetics
Non-Hodgkin's Lymphoma (NHL)
Pharmacokinetics were characterized in 203 NHL patients receiving 375 mg/m2 rituximab weekly by intravenous infusion for 4 doses. Rituximab was detectable in the serum of patients 3 to 6 months after completion of treatment.
The pharmacokinetic profile of rituximab when administered as 6 infusions of 375 mg/m2 in combination with 6 cycles of CHOP chemotherapy was similar to that seen with rituximab alone.
Based on a population pharmacokinetic analysis of data from 298 NHL patients who received rituximab once weekly or once every three weeks, the estimated median terminal elimination half-life was 22 days (range, 6.1 to 52 days). Patients with higher CD19-positive cell counts or larger measurable tumor lesions at pretreatment had a higher clearance. However, dose adjustment for pretreatment CD19 count or size of tumor lesion is not necessary. Age and gender had no effect on the pharmacokinetics of rituximab.
Pharmacokinetics were characterized in 21 patients with CLL receiving rituximab according to the recommended dose and schedule. The estimated median terminal half-life of rituximab was 32 days (range, 14 to 62 days).
Rheumatoid Arthritis
Following administration of 2 doses of rituximab in patients with RA, the mean (±S.D.; % CV) concentrations after the first infusion (Cmax first) and second infusion (Cmax second) were 157 (±46; 29%) and 183 (±55; 30%) mcg/mL, and 318 (±86; 27%) and 381 (±98; 26%) mcg/mL for the 2 × 500 mg and 2 × 1,000 mg doses, respectively.
Based on a population pharmacokinetic analysis of data from 2,005 RA patients who received rituximab, the estimated clearance of rituximab was 0.335 L/day; volume of distribution was 3.1 L and mean terminal elimination half-life was 18.0 days (range, 5.17 to 77.5 days). Age, weight and gender had no effect on the pharmacokinetics of rituximab in RA patients.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis
The pharmacokinetic parameters in adult patients with GPA/MPA receiving 375 mg/m2 intravenous rituximab or non-U.S.-licensed rituximab once weekly for four doses are summarized in Table 4.
Table 4 Population PK in Adult Patients with GPA/MPA Parameter Statistic GPA/MPA Study 1 N
Number of Patients
97
Terminal Half-life
Median
25
(days)
(Range)
(11 to 52)
AUC0–180d
Median
10302
(µg/mL*day)
(Range)
(3653 to 21874)
Clearance
Median
0.279
(L/day)
(Range)
(0.113 to 0.653)
Volume of Distribution
Median
3.12
(L)
(Range)
(2.42 to 3.91)
The population PK analysis in adults with GPA and MPA showed that male patients and patients with higher BSA or positive anti-rituximab antibody levels have higher clearance. However, further dose adjustment based on gender or anti-drug antibody status is not necessary.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term animal studies have been performed to establish the carcinogenic or mutagenic potential of rituximab products or to ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to establish the carcinogenic or mutagenic potential of rituximab products or to determine potential effects on fertility in males or females.
-
14 CLINICAL STUDIES14.1 Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL - The safety and effectiveness of rituximab in relapsed, refractory CD20+ NHL were demonstrated in 3 single-arm ...
14.1 Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in relapsed, refractory CD20+ NHL were demonstrated in 3 single-arm studies enrolling 296 patients.
NHL Study 1
A multicenter, open-label, single-arm study was conducted in 166 patients with relapsed or refractory, low-grade or follicular, B-cell NHL who received 375 mg/m2 of rituximab given as an intravenous infusion weekly for 4 doses. Patients with tumor masses greater than 10 cm or with greater than 5,000 lymphocytes/µL in the peripheral blood were excluded from the study.
Results are summarized in Table 5. The median time to onset of response was 50 days. Disease-related signs and symptoms (including B symptoms) resolved in 64% (25/39) of those patients with such symptoms at study entry.
NHL Study 2
In a multicenter, single-arm study, 37 patients with relapsed or refractory, low-grade NHL received 375 mg/m2 of rituximab weekly for 8 doses. Results are summarized in Table 5.
NHL Study 3
In a multicenter, single-arm study, 60 patients received 375 mg/m2 of rituximab weekly for 4 doses. All patients had relapsed or refractory, low-grade or follicular, B-cell NHL and had achieved an objective clinical response to rituximab administered 3.8–35.6 months (median 14.5 months) prior to retreatment with rituximab. Of these 60 patients, 5 received more than one additional course of rituximab. Results are summarized in Table 5.
Bulky Disease
In pooled data from studies 1 and 3, 39 patients with bulky (single lesion greater than 10 cm in diameter) and relapsed or refractory, low-grade NHL received rituximab 375 mg/m2 weekly for 4 doses. Results are summarized in Table 5.
Table 5 Summary of Rituximab NHL Efficacy Data by Schedule and Clinical Setting NHL Study 1
Weekly × 4
N=166NHL Study 2
Weekly × 8
N=37NHL Study 1 and NHL Study 3
Bulky disease,
Weekly × 4
N=39*NHL Study 3
Retreatment,
Weekly × 4
N=60Overall Response Rate
48%
57%
36%
38%
Complete Response Rate
6%
14%
3%
10%
11.2
[1.9 to 42.1+]13.4
[2.5 to 36.5+]6.9
[2.8 to 25.0+]15.0
[3.0 to 25.1+]14.2 Previously Untreated, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in previously untreated, low-grade or follicular, CD20+ NHL were demonstrated in 3 randomized, controlled trials enrolling 1,662 patients.
NHL Study 4
A total of 322 patients with previously untreated follicular NHL were randomized (1:1) to receive up to eight 3-week cycles of CVP chemotherapy alone (CVP) or in combination with rituximab 375 mg/m2 on Day 1 of each cycle (R-CVP) in an open-label, multicenter study. The main outcome measure of the study was progression-free survival (PFS) defined as the time from randomization to the first of progression, relapse, or death.
Twenty-six percent of the study population was greater than 60 years of age, 99% had Stage III or IV disease, and 50% had an International Prognostic Index (IPI) score greater than or equal to 2. The results for PFS as determined by a blinded, independent assessment of progression are presented in Table 6. The point estimates may be influenced by the presence of informative censoring. The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
NHL Study 5
An open-label, multicenter, randomized (1:1) study was conducted in 1,018 patients with previously untreated follicular NHL who achieved a response (CR or PR) to rituximab in combination with chemotherapy. Patients were randomized to rituximab as single-agent maintenance therapy, 375 mg/m2 every 8 weeks for up to 12 doses or to observation. Rituximab was initiated at 8 weeks following completion of chemotherapy. The main outcome measure of the study was progression-free survival (PFS), defined as the time from randomization in the maintenance/observation phase to progression, relapse, or death, as determined by independent review.
Of the randomized patients, 40% were greater than or equal to 60 years of age, 70% had Stage IV disease, 96% had ECOG performance status (PS) 0–1, and 42% had FLIPI scores of 3–5. Prior to randomization to maintenance therapy, patients had received R-CHOP (75%), R-CVP (22%), or R-FCM (3%); 71% had a complete or unconfirmed complete response and 28% had a partial response.
PFS was longer in patients randomized to rituximab as single-agent maintenance therapy (HR: 0.54, 95% CI: 0.42, 0.70). The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
NHL Study 6
A total of 322 patients with previously untreated low-grade, B-cell NHL who did not progress after 6 or 8 cycles of CVP chemotherapy were enrolled in an open-label, multicenter, randomized trial. Patients were randomized (1:1) to receive rituximab, 375 mg/m2 intravenous infusion, once weekly for 4 doses every 6 months for up to 16 doses or no further therapeutic intervention. The main outcome measure of the study was progression-free survival defined as the time from randomization to progression, relapse, or death. Thirty-seven percent of the study population was greater than 60 years of age, 99% had Stage III or IV disease, and 63% had an IPI score greater than or equal to 2.
There was a reduction in the risk of progression, relapse, or death (hazard ratio estimate in the range of 0.36 to 0.49) for patients randomized to rituximab as compared to those who received no additional treatment.
14.3 Diffuse Large B-Cell NHL (DLBCL)
The safety and effectiveness of rituximab were evaluated in three randomized, active-controlled, open-label, multicenter studies with a collective enrollment of 1,854 patients. Patients with previously untreated diffuse large B-cell NHL received rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
NHL Study 7
A total of 632 patients age greater than or equal to 60 years with DLBCL (including primary mediastinal B‑cell lymphoma) were randomized in a 1:1 ratio to treatment with CHOP or R-CHOP. Patients received 6 or 8 cycles of CHOP, each cycle lasting 21 days. All patients in the R-CHOP arm received 4 doses of rituximab 375 mg/m2 on Days –7 and –3 (prior to Cycle 1) and 48–72 hours prior to Cycles 3 and 5. Patients who received 8 cycles of CHOP also received rituximab prior to Cycle 7. The main outcome measure of the study was progression‑free survival, defined as the time from randomization to the first of progression, relapse, or death. Responding patients underwent a second randomization to receive rituximab or no further therapy.
Among all enrolled patients, 62% had centrally confirmed DLBCL histology, 73% had Stage III–IV disease, 56% had IPI scores greater than or equal to 2, 86% had ECOG performance status of less than 2, 57% had elevated LDH levels, and 30% had two or more extranodal disease sites involved. Efficacy results are presented in Table 7. These results reflect a statistical approach which allows for an evaluation of rituximab administered in the induction setting that excludes any potential impact of rituximab given after the second randomization.
Analysis of results after the second randomization in NHL Study 7 demonstrates that for patients randomized to R-CHOP, additional rituximab exposure beyond induction was not associated with further improvements in progression-free survival or overall survival.
NHL Study 8
A total of 399 patients with DLBCL, age greater than or equal to 60 years, were randomized in a 1:1 ratio to receive CHOP or R-CHOP. All patients received up to eight 3-week cycles of CHOP induction; patients in the R-CHOP arm received rituximab 375 mg/m2 on Day 1 of each cycle. The main outcome measure of the study was event-free survival, defined as the time from randomization to relapse, progression, change in therapy, or death from any cause. Among all enrolled patients, 80% had Stage III or IV disease, 60% of patients had an age-adjusted IPI greater than or equal to 2, 80% had ECOG performance status scores less than 2, 66% had elevated LDH levels, and 52% had extranodal involvement in at least two sites. Efficacy results are presented in Table 7.
NHL Study 9
A total of 823 patients with DLBCL, aged 18–60 years, were randomized in a 1:1 ratio to receive an anthracycline-containing chemotherapy regimen alone or in combination with rituximab. The main outcome measure of the study was time to treatment failure, defined as time from randomization to the earliest of progressive disease, failure to achieve a complete response, relapse, or death. Among all enrolled patients, 28% had Stage III–IV disease, 100% had IPI scores of less than or equal to 1, 99% had ECOG performance status of less than 2, 29% had elevated LDH levels, 49% had bulky disease, and 34% had extranodal involvement. Efficacy results are presented in Table 7.
Table 7 Efficacy Results in NHL Studies 7, 8, and 9 NHL Study 7
(n=632)NHL Study 8
(n=399)NHL Study 9
(n=823)R-CHOP CHOP R-CHOP CHOP R-Chemo Chemo Main outcome Progression-free survival
(years)Event-free survival
(years)Time to treatment failure
(years)Median of main outcome measure
3.1
1.6
2.9
1.1
NE*
NE*
Hazard ratio†
0.69‡
0.60‡
0.45‡
Overall survival at 2 years§
74%
63%
69%
58%
95%
86%
Hazard ratio†
0.72‡
0.68‡
0.40‡
In NHL Study 8, overall survival estimates at 5 years were 58% vs. 46% for R-CHOP and CHOP, respectively.
14.4 Ninety-Minute Infusions in Previously Untreated Follicular NHL and DLBCL
In NHL Study 10, a total of 363 patients with previously untreated follicular NHL (n=113) or DLBCL (n=250) were evaluated in a prospective, open-label, multicenter, single-arm trial for the safety of 90-minute rituximab infusions. Patients with follicular NHL received rituximab 375 mg/m2 plus CVP chemotherapy. Patients with DLBCL received rituximab 375 mg/m2 plus CHOP chemotherapy. Patients with clinically significant cardiovascular disease were excluded from the study. Patients were eligible for a 90-minute infusion at Cycle 2 if they did not experience a Grade 3–4 infusion-related adverse event with Cycle 1 and had a circulating lymphocyte count less than or equal to 5,000/mm3 before Cycle 2. All patients were pre-medicated with acetaminophen and an antihistamine and received the glucocorticoid component of their chemotherapy prior to rituximab infusion. The main outcome measure was the development of Grade 3–4 infusion-related reactions on the day of, or day after, the 90-minute infusion at Cycle 2 [see Adverse Reactions (6.1)].
Eligible patients received their Cycle 2 rituximab infusion over 90 minutes as follows: 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes [see Dosage and Administration (2.1)]. Patients who tolerated the 90-minute rituximab infusion at Cycle 2 continued to receive subsequent rituximab infusions at the 90-minute infusion rate for the remainder of the treatment regimen (through Cycle 6 or Cycle 8).
The incidence of Grade 3–4 infusion-related reactions at Cycle 2 was 1.1% (95% CI [0.3%, 2.8%]) among all patients, 3.5% (95% CI [1.0%, 8.8%]) for those patients treated with R-CVP, and 0.0% (95% CI [0.0%, 1.5%]) for those patients treated with R-CHOP. For Cycles 2–8, the incidence of Grade 3–4 infusion-related reactions was 2.8% (95% CI [1.3%, 5.0%]). No acute fatal infusion-related reactions were observed.
14.5 Chronic Lymphocytic Leukemia (CLL)
The safety and effectiveness of rituximab were evaluated in two randomized (1:1) multicenter open-label studies comparing FC alone or in combination with rituximab for up to 6 cycles in patients with previously untreated CLL [CLL Study 1 (n=817)] or previously treated CLL [CLL Study 2 (n=552)]. Patients received fludarabine 25 mg/m2/day and cyclophosphamide 250 mg/m2/day on days 1, 2, and 3 of each cycle, with or without rituximab. In both studies, seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of rituximab-based therapy.
In CLL Study 1, 30% of patients were 65 years or older, 31% were Binet stage C, 45% had B symptoms, more than 99% had ECOG performance status (PS) 0–1, 74% were male, and 100% were White. In CLL Study 2, 44% of patients were 65 years or older, 28% had B symptoms, 82% received a prior alkylating drug, 18% received prior fludarabine, 100% had ECOG PS 0–1, 67% were male, and 98% were White.
The main outcome measure in both studies was progression-free survival (PFS), defined as the time from randomization to progression, relapse, or death, as determined by investigators (CLL Study 1) or an independent review committee (CLL Study 2). The investigator assessed results in CLL Study 2 were supportive of those obtained by the independent review committee. Efficacy results are presented in Table 8.
Table 8 Efficacy Results in CLL Studies 1 and 2 CLL Study 1*
(Previously untreated)CLL Study 2*
(Previously treated)R-FC
N=408FC
N=409R-FC
N=276FC
N=276- *
- As defined in 1996 National Cancer Institute Working Group guidelines.
Median PFS (months)
39.8
31.5
26.7
21.7
Hazard ratio (95% CI)
0.56 (0.43, 0.71)
0.76 (0.6, 0.96)
P value (Log-Rank test)
<0.01
0.02
Response rate
86%
73%
54%
45%
(95% CI)
(82, 89)
(68, 77)
(48, 60)
(37, 51)
Across both studies, 243 of 676 rituximab-treated patients (36%) were 65 years of age or older and 100 rituximab-treated patients (15%) were 70 years of age or older. The results of exploratory subset analyses in elderly patients are presented in Table 9.
Table 9 Efficacy Results in CLL Studies 1 and 2 in Subgroups Defined by Age* Age subgroup CLL Study 1 CLL Study 2 Number of Patients Hazard Ratio for PFS (95% CI) Number of Patients Hazard Ratio for PFS (95% CI) - *
- From exploratory analyses.
Age less than 65 yrs
572
0.52 (0.39, 0.70)
313
0.61 (0.45, 0.84)
Age greater than or equal to 65 yrs
245
0.62 (0.39, 0.99)
233
0.99 (0.70, 1.40)
Age less than 70 yrs
736
0.51 (0.39, 0.67)
438
0.67 (0.51, 0.87)
Age greater than or equal to 70 yrs
81
1.17 (0.51, 2.66)
108
1.22 (0.73, 2.04)
14.6 Rheumatoid Arthritis (RA)
Reducing the Signs and Symptoms: Initial and Retreatment Courses
The efficacy and safety of rituximab were evaluated in two randomized, double-blind, placebo-controlled studies of adult patients with moderately- to severely-active RA who had a prior inadequate response to at least one TNF inhibitor. Patients were 18 years of age or older, diagnosed with active RA according to American College of Rheumatology (ACR) criteria, and had at least 8 swollen and 8 tender joints.
In RA Study 1 (NCT00468546), patients were randomized to receive either rituximab 2 × 1,000 mg + MTX or placebo + MTX for 24 weeks. Further courses of rituximab 2 × 1,000 mg + MTX were administered in an open‑label extension study at a frequency determined by clinical evaluation, but no sooner than 16 weeks after the preceding course of rituximab. In addition to the intravenous premedication, glucocorticoids were administered orally on a tapering schedule from baseline through Day 14. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24 of the placebo-controlled period are shown in Table 10.
In RA Study 2 (NCT00266227), all patients received the first course of rituximab 2 × 1,000 mg + MTX. Patients who experienced ongoing disease activity were randomized to receive a second course of either rituximab 2 × 1,000 mg + MTX or placebo + MTX, the majority between Weeks 24–28. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24, before the retreatment course, and at Week 48, after retreatment, are shown in Table 10.
Table 10 ACR Responses in RA Study 1 and RA Study 2 (Percent of Patients) (Modified Intent-to-Treat Population) Inadequate Response to TNF Antagonists RA Study 1
24 Week Placebo-Controlled
(Week 24)RA Study 2
Placebo-Controlled Retreatment
(Week 24 and Week 48)Response Placebo + MTX
n=201Rituximab + MTX
n=298Treatment Difference
(Rituximab – Placebo)
(95% CI)Response Placebo + MTX Retreatment
n=157Rituximab + MTX Retreatment
n=318Treatment Difference
(Rituximab – Placebo)*†‡
(95% CI)- *
- In RA Study 2, all patients received a first course of rituximab 2 × 1,000 mg. Patients who experienced ongoing disease activity were randomized to receive a second course of either rituximab 2 × 1,000 mg + MTX or Placebo + MTX at or after Week 24.
- †
- Since all patients received a first course of rituximab, no comparison between Placebo + MTX and rituximab + MTX is made at Week 24.
- ‡
- For RA Study 1, weighted difference stratified by region (US, rest of the world) and Rheumatoid Factor (RF) status (positive greater than 20 IU/mL, negative less than 20 IU/mL) at baseline; For RA Study 2, weighted difference stratified by RF status at baseline and greater than or equal to 20% improvement from baseline in both SJC and TJC at Week 24 (Yes/No).
ACR20
ACR20
Week 24
18%
51%
33%
(26%, 41%)Week 24
48%
45%
NA
Week 48
45%
54%
11%
(2%, 20%)ACR50
ACR50
Week 24
5%
27%
21%
(15%, 27%)Week 24
27%
21%
NA
Week 48
26%
29%
4%
(-4%, 13%)ACR70
ACR70
Week 24
1%
12%
11%
(7%, 15%)Week 24
11%
8%
NA
Week 48
13%
14%
1%
(-5%, 8%)Improvement was also noted for all components of ACR response following treatment with rituximab, as shown in Table 11.
Table 11 Components of ACR Response at Week 24 in RA Study 1 (Modified Intent-to-Treat Population) Inadequate Response to TNF Antagonists Placebo + MTX
(n=201)Rituximab + MTX
(n=298)Parameter
(median)Baseline Wk 24 Baseline Wk 24 Tender Joint Count
31.0
27.0
33.0
13.0
Swollen Joint Count
20.0
19.0
21.0
9.5
Physician Global Assessment*
71.0
69.0
71.0
36.0
Patient Global Assessment*
73.0
68.0
71.0
41.0
Pain*
68.0
68.0
67.0
38.5
Disability Index (HAQ)†
2.0
1.9
1.9
1.5
CRP (mg/dL)
2.4
2.5
2.6
0.9
The time course of ACR 20 response for RA Study 1 is shown in Figure 2. Although both treatment groups received a brief course of intravenous and oral glucocorticoids, resulting in similar benefits at Week 4, higher ACR 20 responses were observed for the rituximab group by Week 8. A similar proportion of patients achieved these responses through Week 24 after a single course of treatment (2 infusions) with rituximab. Similar patterns were demonstrated for ACR 50 and 70 responses.
Radiographic Response
In RA Study 1, structural joint damage was assessed radiographically and expressed as changes in Genant-modified Total Sharp Score (TSS) and its components, the erosion score (ES) and the joint space narrowing (JSN) score. Rituximab + MTX slowed the progression of structural damage compared to placebo + MTX after 1 year as shown in Table 12.
Table 12 Mean Radiographic Change from Baseline to 104 Weeks in RA Study 1 Inadequate Response to TNF Antagonists Parameter Rituximab
2 × 1,000 mg + MTX*Placebo + MTX† Treatment Difference
(Placebo – Rituximab)95% CI Change during First Year
TSS
0.66
1.77
1.11
(0.47, 1.75)
ES
0.44
1.19
0.75
(0.32, 1.19)
JSN Score
0.22
0.58
0.36
(0.10, 0.62)
Change during Second Year‡
TSS
0.48
1.04
—
—
ES
0.28
0.62
—
—
JSN Score
0.20
0.42
—
—
In RA Study 1 and its open-label extension, 70% of patients initially randomized to rituximab + MTX and 72% of patients initially randomized to placebo + MTX were evaluated radiographically at Year 2. As shown in Table 12, progression of structural damage in rituximab + MTX patients was further reduced in the second year of treatment.
Following 2 years of treatment with rituximab + MTX, 57% of patients had no progression of structural damage. During the first year, 60% of rituximab + MTX treated patients had no progression, defined as a change in TSS of zero or less compared to baseline, compared to 46% of placebo + MTX treated patients. In their second year of treatment with rituximab + MTX, more patients had no progression than in the first year (68% vs. 60%), and 87% of the rituximab + MTX treated patients who had no progression in the first year also had no progression in the second year.
Lesser Efficacy of 500 vs. 1,000 mg Treatment Courses for Radiographic Outcomes
RA Study 3 (NCT00299104) is a randomized, double-blind, placebo-controlled study which evaluated the effect of placebo + MTX compared to rituximab 2 × 500 mg + MTX and rituximab 2 × 1,000 mg + MTX treatment courses in MTX-naïve RA patients with moderately-to severely-active disease. Patients received a first course of two infusions of rituximab or placebo on Days 1 and 15. MTX was initiated at 7.5 mg/week and escalated up to 20 mg/week by Week 8 in all three treatment arms. After a minimum of 24 weeks, patients with ongoing disease activity were eligible to receive retreatment with additional courses of their assigned treatment. After one year of treatment, the proportion of patients achieving ACR 20/50/70 responses were similar in both rituximab dose groups and were higher than in the placebo group. However, with respect to radiographic scores, only the rituximab 1,000 mg treatment group demonstrated a statistically significant reduction in TSS: a change of 0.36 units compared to 1.08 units for the placebo group, a 67% reduction.
Physical Function Response
RA Study 4 (NCT00299130) is a randomized, double-blind, placebo-controlled study in adult RA patients with moderately-to severely-active disease with inadequate response to MTX. Patients were randomized to receive an initial course of rituximab 500 mg, rituximab 1,000 mg, or placebo in addition to background MTX.
Physical function was assessed at Weeks 24 and 48 using the Health Assessment Questionnaire Disability Index (HAQ-DI). From baseline to Week 24, a greater proportion of rituximab-treated patients had an improvement in HAQ-DI of at least 0.22 (a minimal clinically important difference) and a greater mean HAQ-DI improvement compared to placebo, as shown in Table 13. HAQ-DI results for the rituximab 500 mg treatment group were similar to the rituximab 1,000 mg treatment group; however radiographic responses were not assessed (see Dosing Precaution in the Radiographic Responses section above). These improvements were maintained at 48 weeks.
Table 13 Improvement from Baseline in Health Assessment Questionnaire Disability Index (HAQ-DI) at Week 24 in RA Study 4 Placebo + MTX
n=172Rituximab
2 × 1,000 mg + MTX
n=170Treatment Difference
(Rituximab - Placebo)*
(95% CI)Mean Improvement from Baseline
0.19
0.42
0.23 (0.11, 0.34)
Percent of patients with "Improved" score
(Change from Baseline greater than or equal to MCID)†48%
58%
11% (0%, 21%)
Close14.7 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Induction Treatment of Adult Patients with Active Disease (GPA/MPA Study 1)
A total of 197 patients with active, severe GPA and MPA (two forms of ANCA‑Associated Vasculitides) were treated in a randomized, double-blind, active-controlled multicenter, non-inferiority study, conducted in two phases – a 6 month remission induction phase and a 12 month remission maintenance phase. Patients were 15 years of age or older, diagnosed with GPA (75% of patients) or MPA (24% of patients) according to the Chapel Hill Consensus conference criteria (1% of the patients had unknown vasculitis type). All patients had active disease, with a Birmingham Vasculitis Activity Score for Granulomatosis with Polyangiitis (BVAS/GPA) greater than or equal to 3, and their disease was severe, with at least one major item on the BVAS/GPA. Ninety-six (49%) of patients had new disease and 101 (51%) of patients had relapsing disease.
Patients in both arms received 1,000 mg of pulse intravenous methylprednisolone per day for 1 to 3 days within 14 days prior to initial infusion. Patients were randomized in a 1:1 ratio to receive either rituximab 375 mg/m2 once weekly for 4 weeks or oral cyclophosphamide 2 mg/kg daily for 3 to 6 months in the remission induction phase. Patients were pre-medicated with antihistamine and acetaminophen prior to rituximab infusion. Following intravenous corticosteroid administration, all patients received oral prednisone (1 mg/kg/day, not exceeding 80 mg/day) with pre-specified tapering. Once remission was achieved or at the end of the 6 month remission induction period, the cyclophosphamide group received azathioprine to maintain remission. The rituximab group did not receive additional therapy to maintain remission. The main outcome measure for both GPA and MPA patients was achievement of complete remission at 6 months defined as a BVAS/GPA of 0, and off glucocorticoid therapy. The pre-specified non-inferiority margin was a treatment difference of 20%. As shown in Table 14, the study demonstrated non-inferiority of rituximab to cyclophosphamide for complete remission at 6 months.
Complete Remission (CR) at 12 and 18 Months
In the rituximab group, 44% of patients achieved CR at 6 and 12 months, and 38% of patients achieved CR at 6, 12, and 18 months. In patients treated with cyclophosphamide (followed by azathioprine for maintenance of CR), 38% of patients achieved CR at 6 and 12 months, and 31% of patients achieved CR at 6, 12, and 18 months.
Retreatment of Flares with Rituximab
Based upon investigator judgment, 15 patients received a second course of rituximab therapy for treatment of relapse of disease activity which occurred between 8 and 17 months after the induction treatment course of rituximab.
Follow up Treatment of Adult Patients with GPA/MPA who have achieved disease control with other Immunosuppressant (GPA/MPA Study 2)
A total of 115 patients (86 with GPA, 24 with MPA, and 5 with renal-limited ANCA-associated vasculitis) in disease remission were randomized to receive azathioprine (58 patients) or non-U.S.-licensed rituximab (57 patients) in this open-label, prospective, multicenter, randomized, active-controlled study. Eligible patients were 21 years and older and had either newly diagnosed (80%) or relapsing disease (20%). A majority of the patients were ANCA-positive. Remission of active disease was achieved using a combination of glucocorticoids and cyclophosphamide. Within a maximum of 1 month after the last cyclophosphamide dose, eligible patients (based on BVAS of 0), were randomized in a 1:1 ratio to receive either non-U.S.-licensed rituximab or azathioprine.
The non-U.S.-licensed rituximab was administered as two 500 mg intravenous infusions separated by two weeks (on Day 1 and Day 15) followed by a 500 mg intravenous infusion every 6 months for 18 months. Azathioprine was administered orally at a dose of 2 mg/kg/day for 12 months, then 1.5 mg/kg/day for 6 months, and finally 1 mg/kg/day for 4 months; treatment was discontinued after 22 months. Prednisone treatment was tapered and then kept at a low dose (approximately 5 mg per day) for at least 18 months after randomization. Prednisone dose tapering and the decision to stop prednisone treatment after month 18 were left at the investigator's discretion.
Planned follow-up was until month 28 (10 or 6 months, respectively, after the last non-U.S.-licensed rituximab infusion or azathioprine dose). The primary endpoint was the occurrence of major relapse (defined by the reappearance of clinical and/or laboratory signs of vasculitis activity that could lead to organ failure or damage, or could be life-threatening) through month 28.
By month 28, major relapse occurred in 3 patients (5%) in the non-U.S.-licensed rituximab group and 17 patients (29%) in the azathioprine group.
The observed cumulative incidence rate of first major relapse during the 28 months was lower in patients on non-U.S.-licensed rituximab relative to azathioprine (Figure 3).
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRUXIENCE (rituximab-pvvr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale brownish-yellow solution for intravenous infusion supplied as ...
RUXIENCE (rituximab-pvvr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale brownish-yellow solution for intravenous infusion supplied as follows:
Carton contents NDC number One 100 mg/10 mL (10 mg/mL) single-dose vial
NDC 0069-0238-01
One 500 mg/50 mL (10 mg/mL) single-dose vial
NDC 0069-0249-01
CloseStore RUXIENCE vials refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton. RUXIENCE vials should be protected from direct sunlight. Do not freeze or shake.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Infusion-Related Reactions - Inform patients about the signs and symptoms of infusion-related reactions. Advise ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Infusion-Related Reactions
Inform patients about the signs and symptoms of infusion-related reactions. Advise patients to contact their healthcare provider immediately to report symptoms of infusion-related reactions including urticaria, hypotension, angioedema, sudden cough, breathing problems, weakness, dizziness, palpitations, or chest pain [see Warnings and Precautions (5.1)].
Severe Mucocutaneous Reactions
Advise patients to contact their healthcare provider immediately for symptoms of severe mucocutaneous reactions, including painful sores or ulcers on the mouth, blisters, peeling skin, rash, and pustules [see Warnings and Precautions (5.2)].
Hepatitis B Virus Reactivation
Advise patients to contact their healthcare provider immediately for symptoms of hepatitis including worsening fatigue or yellow discoloration of skin or eyes [see Warnings and Precautions (5.3)].
Progressive Multifocal Leukoencephalopathy (PML)
Advise patients to contact their healthcare provider immediately for signs and symptoms of PML, including new or changes in neurological symptoms such as confusion, dizziness or loss of balance, difficulty talking or walking, decreased strength or weakness on one side of the body, or vision problems [see Warnings and Precautions (5.4)].
Tumor Lysis Syndrome (TLS)
Advise patients to contact their healthcare provider immediately for signs and symptoms of tumor lysis syndrome such as nausea, vomiting, diarrhea, and lethargy [see Warnings and Precautions (5.5)].
Infections
Advise patients to contact their healthcare provider immediately for signs and symptoms of infections including fever, cold symptoms (e.g., rhinorrhea or laryngitis), flu symptoms (e.g., cough, fatigue, body aches), earache or headache, dysuria, oral herpes simplex infection, and painful wounds with erythema and advise patients of the increased risk of infections during and after treatment with RUXIENCE [see Warnings and Precautions (5.6)].
Cardiovascular Adverse Reactions
Advise patients of the risk of cardiovascular adverse reactions, including ventricular fibrillation, myocardial infarction, and cardiogenic shock. Advise patients to contact their healthcare provider immediately to report chest pain and irregular heartbeats [see Warnings and Precautions (5.7)].
Renal Toxicity
Advise patients of the risk of renal toxicity. Inform patients of the need for healthcare providers to monitor kidney function [see Warnings and Precautions (5.8)].
Bowel Obstruction and Perforation
Advise patients to contact their healthcare provider immediately for signs and symptoms of bowel obstruction and perforation, including severe abdominal pain or repeated vomiting [see Warnings and Precautions (5.9)].
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.11), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with RUXIENCE and for 12 months after the last dose [see Use in Specific Populations (8.3)].
CloseLactation
Advise women not to breastfeed during treatment with RUXIENCE and for 6 months after the last dose [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTIONManufactured by - Pfizer Ireland Pharmaceuticals - Cork, Ireland, P43 X336 - U.S. License No. 2060 - Distributed by - Pfizer Labs - Division of Pfizer Inc. New York, NY 10001 - This product’s labeling may have ...
Manufactured by
Pfizer Ireland Pharmaceuticals
Cork, Ireland, P43 X336
U.S. License No. 2060Distributed by
Pfizer Labs
Division of Pfizer Inc. New York, NY 10001
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-1273-4.0
Close -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration - Revised: 10/2023 - MEDICATION GUIDE - RUXIENCE® (RUKSee-ents) (rituximab-pvvr) injection - What is ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised: 10/2023
MEDICATION GUIDE
RUXIENCE® (RUKSee-ents)
(rituximab-pvvr)
injectionWhat is the most important information I should know about RUXIENCE?
RUXIENCE can cause serious side effects that can lead to death, including:- •
-
Infusion-related reactions. Infusion-related reactions are very common side effects of RUXIENCE treatment. Serious infusion-related reactions can happen during your infusion or within 24 hours after your infusion of RUXIENCE. Your healthcare provider should give you medicines before your infusion of RUXIENCE to decrease your chance of having a severe infusion-related reaction.
Tell your healthcare provider or get medical help right away if you get any of these symptoms during or after an infusion of RUXIENCE:
- o
- hives (red itchy welts) or rash
- o
- itching
- o
- swelling of your lips, tongue, throat or face
- o
- sudden cough
- o
- shortness of breath, difficulty breathing, or wheezing
- o
- weakness
- o
- dizziness or feel faint
- o
- palpitations (feel like your heart is racing or fluttering)
- o
- chest pain
- •
-
Severe skin and mouth reactions. Tell your healthcare provider or get medical help right away if you get any of these symptoms at any time during your treatment with RUXIENCE:
- o
- painful sores or ulcers on your skin, lips or in your mouth
- o
- blisters
- o
- peeling skin
- o
- rash
- o
- pustules
- •
-
Hepatitis B virus (HBV) reactivation. Before you receive your RUXIENCE treatment, your healthcare provider will do blood tests to check for HBV infection. If you have had hepatitis B or are a carrier of hepatitis B virus, receiving RUXIENCE could cause the virus to become an active infection again. Hepatitis B reactivation may cause serious liver problems including liver failure, and death. You should not receive RUXIENCE if you have active hepatitis B liver disease. Your healthcare provider will monitor you for hepatitis B infection during and for several months after you stop receiving RUXIENCE.
Tell your healthcare provider right away if you get worsening tiredness, or yellowing of your skin or white part of your eyes, during treatment with RUXIENCE. - •
-
Progressive Multifocal Leukoencephalopathy (PML). PML is a rare, serious brain infection caused by a virus that can happen in people who receive RUXIENCE. People with weakened immune systems can get PML. PML can result in death or severe disability. There is no known treatment, prevention, or cure for PML.
Tell your healthcare provider right away if you have any new or worsening symptoms or if anyone close to you notices these symptoms:
- o
- confusion
- o
- dizziness or loss of balance
- o
- difficulty walking or talking
- o
- decreased strength or weakness on one side of your body
- o
- vision problems
See "What are the possible side effects of RUXIENCE?" for more information about side effects.
What is RUXIENCE?
RUXIENCE is a prescription medicine used to treat:- •
- Adults with Non-Hodgkin's Lymphoma (NHL): alone or with other chemotherapy medicines.
- •
- Adults with Chronic Lymphocytic Leukemia (CLL): with the chemotherapy medicines fludarabine and cyclophosphamide.
- •
- Adults with Rheumatoid Arthritis (RA): with another prescription medicine called methotrexate, to reduce the signs and symptoms of moderate to severe active RA in adults, after treatment with at least one other medicine called a Tumor Necrosis Factor (TNF) antagonist has been used and did not work well.
- •
- Adults with Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA): with glucocorticoids, to treat GPA and MPA.
RUXIENCE is not indicated for treatment of children.
Before you receive RUXIENCE, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have had a severe reaction to RUXIENCE or another rituximab product
- •
- have a history of heart problems, irregular heart beat or chest pain
- •
- have lung or kidney problems
- •
- have an infection or weakened immune system
- •
- have or have had any severe infections including:
- o
- Hepatitis B virus (HBV)
- o
- Hepatitis C virus (HCV)
- o
- Cytomegalovirus (CMV)
- o
- Herpes simplex virus (HSV)
- o
- Parvovirus B19
- o
- Varicella zoster virus (chickenpox or shingles)
- o
- West Nile Virus
- •
- have had a recent vaccination or are scheduled to receive vaccinations. You should not receive certain vaccines before or during treatment with RUXIENCE.
- •
- are pregnant or plan to become pregnant. Talk to your healthcare provider about the risks to your unborn baby if you receive RUXIENCE during pregnancy.
Females who are able to become pregnant:- o
- Your healthcare provider should do a pregnancy test to see if you are pregnant before starting RUXIENCE.
- o
- You should use effective birth control (contraception) during treatment with RUXIENCE and for 12 months after your last dose of RUXIENCE. Talk to your healthcare provider about effective birth control.
- o
- Tell your healthcare provider right away if you become pregnant or think that you are pregnant during treatment with RUXIENCE.
- •
- are breastfeeding or plan to breastfeed. RUXIENCE may pass into your breast milk. Do not breastfeed during treatment and for 6 months after your last dose of RUXIENCE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take or have taken:
- •
- a Tumor Necrosis Factor (TNF) inhibitor medicine
- •
- a Disease Modifying Anti-Rheumatic Drug (DMARD)
If you are not sure if your medicine is one listed above, ask your healthcare provider.
How will I receive RUXIENCE?
- •
- RUXIENCE is given by infusion through your central catheter or through a needle placed in a vein (intravenous infusion), in your arm. Talk to your healthcare provider about how you will receive RUXIENCE.
- •
- Your healthcare provider may prescribe medicines before each infusion of RUXIENCE to reduce infusion side effects such as fever and chills.
- •
- Your healthcare provider should do blood tests regularly to check for side effects to RUXIENCE.
- •
- Before each RUXIENCE treatment, your healthcare provider or nurse will ask you questions about your general health. Tell your healthcare provider or nurse about any new symptoms.
What are the possible side effects of RUXIENCE?
RUXIENCE can cause serious side effects, including:- •
- See "What is the most important information I should know about RUXIENCE?"
- •
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause you to have:
- o
- kidney failure and the need for dialysis treatment
- o
- abnormal heart rhythm
- TLS can happen within 12 to 24 hours after an infusion of RUXIENCE. Your healthcare provider may do blood tests to check you for TLS. Your healthcare provider may give you medicine to help prevent TLS.
Tell your healthcare provider right away if you have any of the following signs or symptoms of TLS:
- o
- nausea
- o
- vomiting
- o
- diarrhea
- o
- lack of energy
- •
-
Serious infections. Serious infections can happen during and after treatment with RUXIENCE, and can lead to death. RUXIENCE can increase your risk of getting infections and can lower the ability of your immune system to fight infections. Types of serious infections that can happen with RUXIENCE include bacterial, fungal, and viral infections. After receiving RUXIENCE, some people have developed low levels of certain antibodies in their blood for a long period of time (longer than 11 months). Some of these people with low antibody levels developed infections. People with serious infections should not receive RUXIENCE. Tell your healthcare provider right away if you have any symptoms of infection:
- o
- fever
- o
- cold symptoms, such as runny nose or sore throat that do not go away
- o
- flu symptoms, such as cough, tiredness, and body aches
- o
- earache or headache
- o
- pain during urination
- o
- cold sores in the mouth or throat
- o
- cuts, scrapes or incisions that are red, warm, swollen or painful
- •
- Heart problems. RUXIENCE may cause chest pain, irregular heartbeats, and heart attack. Your healthcare provider may monitor your heart during and after treatment with RUXIENCE if you have symptoms or heart problems or have a history of heart problems. Tell your healthcare provider right away if you have chest pain or irregular heartbeats during treatment with RUXIENCE.
- •
- Kidney problems, especially if you are receiving RUXIENCE for NHL. RUXIENCE can cause severe kidney problems that lead to death. Your healthcare provider should do blood tests to check how well your kidneys are working.
- •
- Stomach and Serious bowel problems that can sometimes lead to death. Bowel problems, including blockage or tears in the bowel can happen if you receive RUXIENCE with chemotherapy medicines. Tell your healthcare provider right away if you have any severe stomach-area (abdomen) pain or repeated vomiting during treatment with RUXIENCE.
Your healthcare provider will stop treatment with RUXIENCE if you have severe, serious or life-threatening side effects.
The most common side effects of RUXIENCE include:- o
- infusion-related reactions (see "What is the most important information I should know about RUXIENCE?")
- o
- infections (may include fever, chills)
- o
- body aches
- o
- tiredness
- o
- nausea
In adults with GPA or MPA the most common side effects of RUXIENCE also include:
- o
- low white and red blood cells
- o
- swelling
- o
- diarrhea
- o
- muscle spasms
Other side effects with RUXIENCE include:
- o
- aching joints during or within hours of receiving an infusion
- o
- more frequent upper respiratory tract infection
These are not all of the possible side effects with RUXIENCE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of RUXIENCE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about RUXIENCE that is written for healthcare providers.What are the ingredients in RUXIENCE?
Active ingredient: rituximab-pvvr
Inactive ingredients: edetate disodium dihydrate, L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sucrose, and Water for Injection.Manufactured by Pfizer Ireland Pharmaceuticals, Cork, Ireland, P43 X336
U.S. License No. 2060
Distributed by Pfizer Labs Division of Pfizer Inc. New York, NY 10001
LAB-1274-4.0

For more information, go to www.pfizer.com or call 1-800-438-1985. -
PRINCIPAL DISPLAY PANEL - 10 mL Vial LabelALWAYS DISPENSE ENCLOSED - MEDICATION GUIDE TO EACH PATIENT - NDC 0069-0238-01 - Rx only - Ruxience® (rituximab-pvvr) Injection - 100 mg/10 mL - (10 mg/mL) For Intravenous Use after dilution - ONE SINGLE-DOSE ...
ALWAYS DISPENSE ENCLOSED
MEDICATION GUIDE TO EACH PATIENTNDC 0069-0238-01
Rx onlyRuxience®
(rituximab-pvvr)
Injection100 mg/10 mL
(10 mg/mL)For Intravenous Use after dilution
ONE SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Close -
PRINCIPAL DISPLAY PANEL - 10 mL Vial CartonALWAYS DISPENSE ENCLOSED - MEDICATION GUIDE TO EACH PATIENT - NDC 0069-0238-01 - Ruxience® (rituximab-pvvr) Injection - 100 mg/10 mL - (10 mg/mL) For Intravenous Use - after dilution - ONE SINGLE-DOSE ...
ALWAYS DISPENSE ENCLOSED
MEDICATION GUIDE TO EACH PATIENTNDC 0069-0238-01
Ruxience®
(rituximab-pvvr)
Injection100 mg/10 mL
(10 mg/mL)For Intravenous Use
after dilutionONE SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Rx only
Pfizer Oncology
Close -
PRINCIPAL DISPLAY PANEL - 50 mL Vial LabelALWAYS DISPENSE ENCLOSED MEDICATION GUIDE TO EACH PATIENT - NDC 0069-0249-01 - Ruxience® (rituximab-pvvr) Injection - 500 mg/50 mL - (10 mg/mL) For Intravenous Use after dilution - ONE SINGLE-DOSE ...
ALWAYS DISPENSE ENCLOSED MEDICATION GUIDE TO EACH PATIENT
NDC 0069-0249-01
Ruxience®
(rituximab-pvvr)
Injection500 mg/50 mL
(10 mg/mL)For Intravenous Use after dilution
ONE SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Rx only
Close -
PRINCIPAL DISPLAY PANEL - 50 mL Vial CartonALWAYS DISPENSE ENCLOSED - MEDICATION GUIDE TO EACH PATIENT - NDC 0069-0249-01 - Ruxience® (rituximab-pvvr) Injection - 500 mg/50 mL - (10 mg/mL) For Intravenous Use - after dilution - ONE SINGLE-DOSE ...
ALWAYS DISPENSE ENCLOSED
MEDICATION GUIDE TO EACH PATIENTNDC 0069-0249-01
Ruxience®
(rituximab-pvvr)
Injection500 mg/50 mL
(10 mg/mL)For Intravenous Use
after dilutionONE SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Rx only
Pfizer Oncology
Close -
INGREDIENTS AND APPEARANCEProduct Information
RUXIENCE rituximab-pvvr injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0238 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITUXIMAB (UNII: 4F4X42SYQ6) (RITUXIMAB - UNII:4F4X42SYQ6) RITUXIMAB 100 mg in 10 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 25.7 mg in 10 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 2 mg in 10 mL SUCROSE (UNII: C151H8M554) 850 mg in 10 mL WATER (UNII: 059QF0KO0R) HISTIDINE (UNII: 4QD397987E) 12 mg in 10 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 25.7 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0238-01 1 in 1 CARTON 01/23/2020 1 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761103 01/23/2020 RUXIENCE rituximab-pvvr injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0249 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITUXIMAB (UNII: 4F4X42SYQ6) (RITUXIMAB - UNII:4F4X42SYQ6) RITUXIMAB 500 mg in 50 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 128.5 mg in 50 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 10 mg in 50 mL SUCROSE (UNII: C151H8M554) 4250 mg in 50 mL WATER (UNII: 059QF0KO0R) HISTIDINE (UNII: 4QD397987E) 60 mg in 50 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 128.5 mg in 50 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0249-01 1 in 1 CARTON 01/23/2020 1 50 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761103 01/23/2020 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 985586408 ANALYSIS(0069-0238, 0069-0249) , API MANUFACTURE(0069-0238, 0069-0249) Establishment Name Address ID/FEI Business Operations Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC 174350868 API MANUFACTURE(0069-0238, 0069-0249)
CloseEstablishment Name Address ID/FEI Business Operations Pfizer Manufacturing Belgium NV 370156507 ANALYSIS(0069-0238, 0069-0249) , MANUFACTURE(0069-0238, 0069-0249) , PACK(0069-0238, 0069-0249) , LABEL(0069-0238, 0069-0249)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
RUXIENCE- rituximab-pvvr injection, solution
Number of versions: 8
RxNorm
RUXIENCE- rituximab-pvvr injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2273512 | riTUXimab-pvvr 100 MG in 10 ML Injection | PSN |
| 2 | 2273512 | 10 ML rituximab-pvvr 10 MG/ML Injection | SCD |
| 3 | 2273512 | rituximab-pvvr 100 MG per 10 ML Injection | SY |
| 4 | 2273517 | Ruxience 100 MG in 10 ML Injection | PSN |
| 5 | 2273517 | 10 ML rituximab-pvvr 10 MG/ML Injection [Ruxience] | SBD |
| 6 | 2273517 | 10 ML Ruxience 10 MG/ML Injection | SY |
| 7 | 2273517 | Ruxience 100 MG per 10 ML Injection | SY |
| 8 | 2273520 | riTUXimab-pvvr 500 MG in 50 ML Injection | PSN |
| 9 | 2273520 | 50 ML rituximab-pvvr 10 MG/ML Injection | SCD |
| 10 | 2273520 | rituximab-pvvr 500 MG per 50 ML Injection | SY |
| 11 | 2273521 | Ruxience 500 MG in 50 ML Injection | PSN |
| 12 | 2273521 | 50 ML rituximab-pvvr 10 MG/ML Injection [Ruxience] | SBD |
| 13 | 2273521 | 50 ML Ruxience 10 MG/ML Injection | SY |
| 14 | 2273521 | Ruxience 500 MG per 50 ML Injection | SY |
Get Label RSS Feed for this Drug
RUXIENCE- rituximab-pvvr injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=f941fc61-f7a3-4e4a-ab7c-87c1667fa05b
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
RUXIENCE- rituximab-pvvr injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0069-0238-01 |
| 2 | 0069-0249-01 |