Label: ROCKLATAN- netarsudil and latanoprost ophthalmic solution, 0.02%/0.005% solution/ drops
- NDC Code(s): 70727-529-25, 70727-529-99
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ROCKLATAN® safely and effectively. See full prescribing information for ROCKLATAN®. ROCKLATAN® (netarsudil and latanoprost ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGE ROCKLATAN is a fixed dose combination of a Rho kinase inhibitor and a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle ...
-
2. DOSAGE AND ADMINISTRATION The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose in the evening. The dosage of ROCKLATAN ...
-
3. DOSAGE FORMS AND STRENGTHS Ophthalmic solution containing netarsudil 0.02% (0.2 mg/mL) and latanoprost 0.005% (0.05 mg/mL).
-
4. CONTRAINDICATIONS None.
-
5. WARNINGS AND PRECAUTIONS 5.1 Epithelial Corneal Edema - ROCKLATAN contains netarsudil which has been associated with Epithelial corneal edema, described as honeycomb or bullous, and has been reported in some patients ...
-
6. ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7. DRUG INTERACTIONS In vitro drug interaction studies have shown that precipitation can occur when eye drops containing thimerosal are mixed with ROCKLATAN. If such drugs are used, they should be administered at ...
-
8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of ROCKLATAN ophthalmic solution or its pharmacologically active ingredients (netarsudil and latanoprost) in ...
-
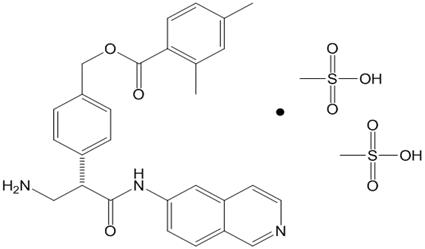
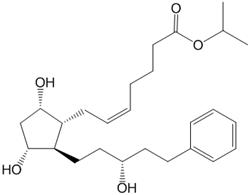
11. DESCRIPTION ROCKLATAN (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is a fixed dose combination of a Rho kinase inhibitor and a prostaglandin F2α analogue. The chemical name of netarsudil ...
-
12. CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - ROCKLATAN is comprised of two components: netarsudil and latanoprost. Each of these two components decreases elevated IOP. Elevated IOP represents a major risk factor ...
-
13. NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of ...
-
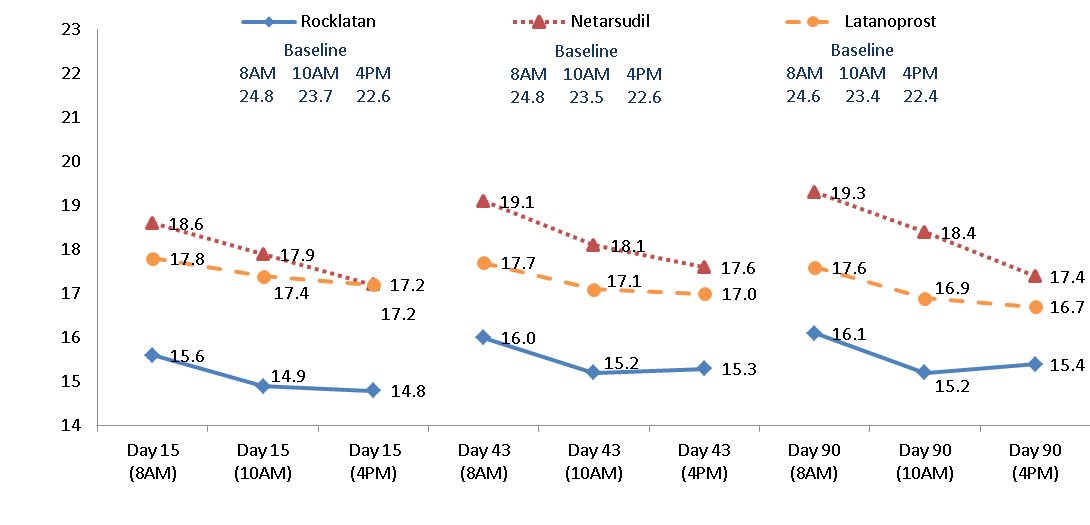
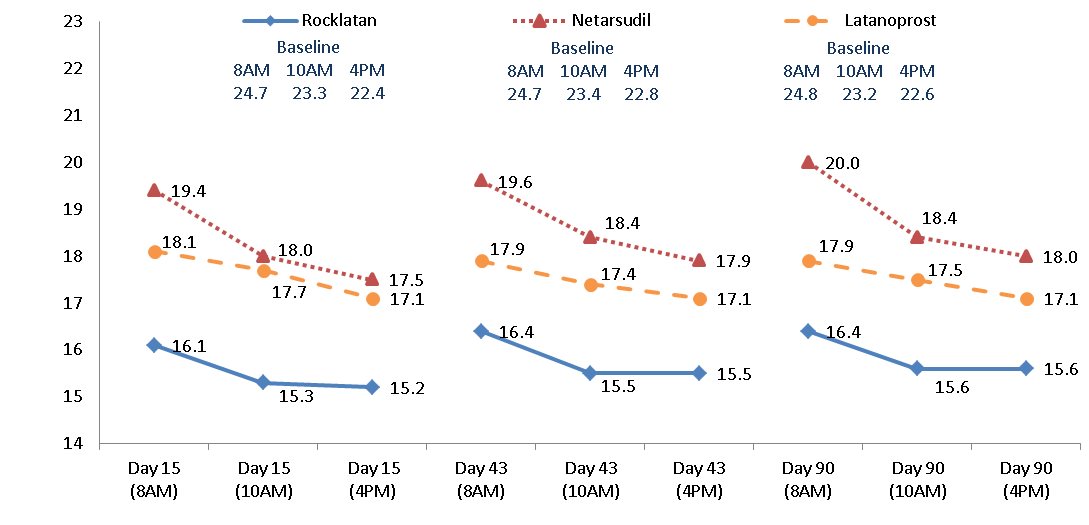
14. CLINICAL STUDIES ROCKLATAN (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% was evaluated in 2 randomized and controlled clinical trials, namely PG324-CS301 (NCT 02558400, referred to as Study 301 ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING ROCKLATAN (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is supplied sterile in clear low density polyethylene bottles with opaque white polyethylene dropper tips and white ...
-
17. PATIENT COUNSELING INFORMATION Potential for Pigmentation - Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin ...
-
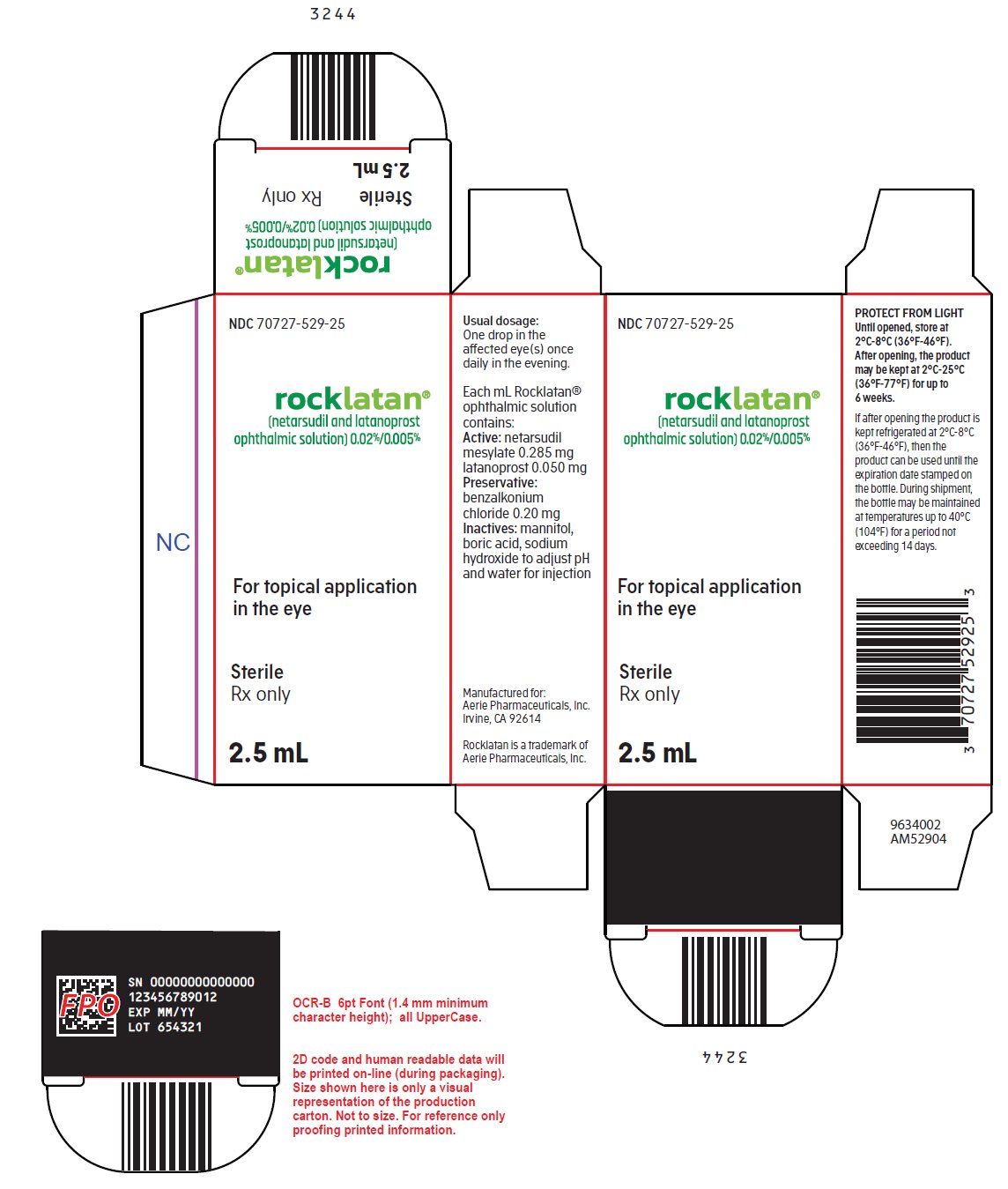
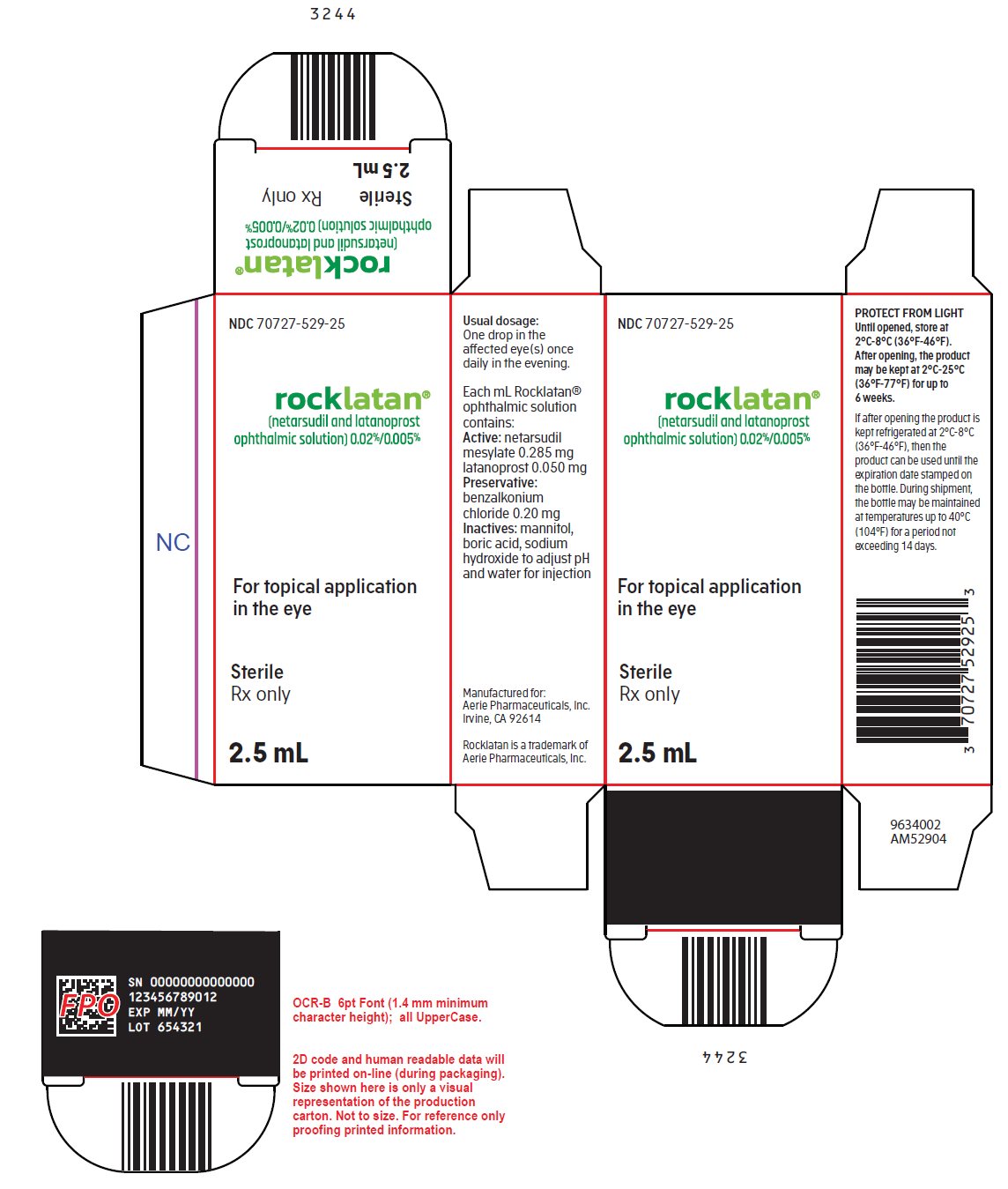
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 70727-529-25 - rocklatan® (netarsudil and latanoprost - ophthalmic solution) 0.02%/0.005% For topical application - in the eye - Sterile - Rx only - 2.5 mL - Usual dosage: One drop ...
-
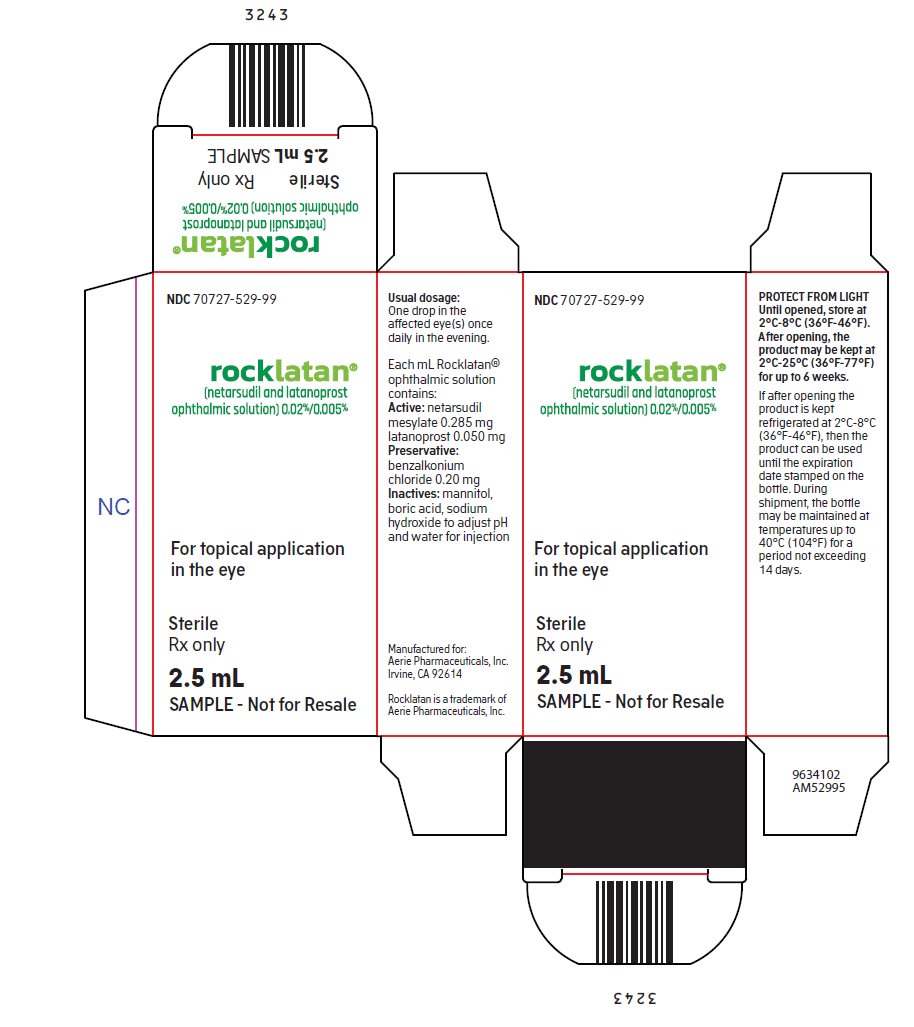
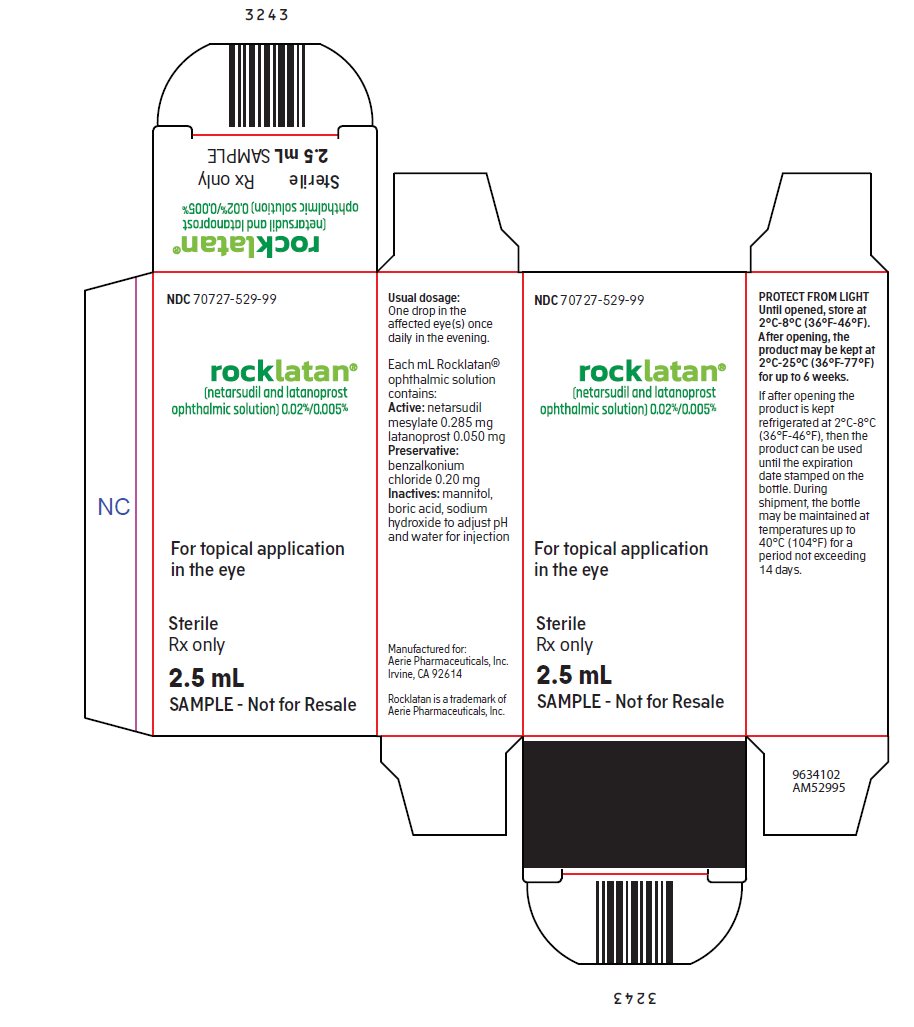
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 70727-529-99 - rocklatan® (netarsudil and latanoprost - ophthalmic solution) 0.02%/0.005% For topical application - in the eye - Sterile - Rx only - 2.5 mL - SAMPLE – Not for Resale
-
INGREDIENTS AND APPEARANCEProduct Information