Label: RIOMET- metformin hydrochloride solution
- NDC Code(s): 10631-206-01, 10631-206-02, 10631-238-01, 10631-238-02
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RIOMET safely and effectively. See full prescribing information for RIOMET. RIOMET® (metformin hydrochloride) solution, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin‑ associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin‑ associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue RIOMET and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGERIOMET is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus.
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Dosage - • Measure the RIOMET dose in the RIOMET specific dosing cup. • The recommended starting dose of RIOMET is 500 mg (5 mL) orally twice a day or 850 mg (8.5 mL) once a day, given ...
-
3 DOSAGE FORMS AND STRENGTHSOral solution: 500 mg per 5 mL (100 mg/mL) clear solution in cherry and strawberry flavor
-
4 CONTRAINDICATIONS1. RIOMET is contraindicated in patients with: 2. Severe renal impairment (eGFR below 30 mL/min/1.73 m2) [see Warnings and Precautions (5.1)]. 3. Hypersensitivity to metformin. 4. Acute or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Lactic Acidosis - • There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific ...
-
6 ADVERSE REACTIONS• The following adverse reactions are also discussed elsewhere in the labeling: • Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)] • Vitamin B12 Deficiency [see Warnings ...
-
7 DRUG INTERACTIONS1. Table 2 presents clinically significant drug interactions with RIOMET. 2. Table 2: Clinically Significant Drug Interactions with RIOMET - 1. Carbonic Anhydrase ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - 1. Risk Summary - 2. Limited data with RIOMET in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. Published studies ...
-
10 OVERDOSAGE• Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with ...
-
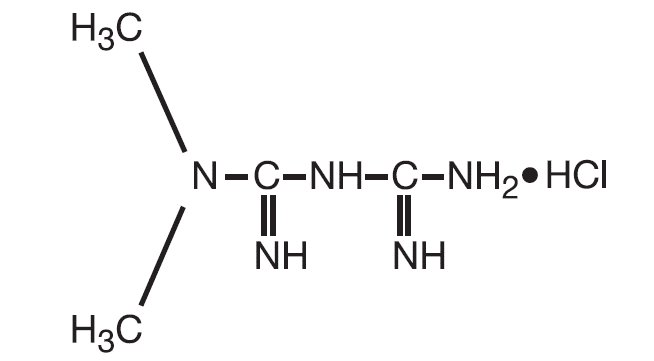
11 DESCRIPTIONRIOMET oral solution contains the biguanidine antihyperglycemic agent metformin in the form of monohydrochloride salt. Metformin hydrochloride, is N,N-dimethylimidodicarbonimidic diamide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - • Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - 1. Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks ...
-
14 CLINICAL STUDIES1. Adult Clinical Studies - 2. A double-blind, placebo-controlled, multicenter US clinical trial involving obese patients with type - 3. 2 diabetes mellitus whose hyperglycemia was not adequately ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - 1. RIOMET 500 mg per 5 mL (100 mg/mL) oral solution is supplied in bottles with child-resistant caps and a dosing cup as ...
-
17 PATIENT COUNSELING INFORMATION1. Advise the patient to read the FDA-approved patient labeling (Patient Information). 2. Administration: 3. Instruct patients or caregivers to use the supplied dosing cup to measure the ...
-
Patient Medication Information • PATIENT INFORMATION - • RIOMET (ree oh met) • (metformin hydrochloride) • oral solution - • What is the most important information I should know about RIOMET? • RIOMET can cause serious ...
-
PACKAGE LABEL. PRINCIPAL DISPLAY PANELNDC 10631-238-02 - Riomet® (metformin hydrochloride oral solution) 500 mg/5 mL - Each 5 mL contains: 500 mg of metformin hydrochloride, USP. Strawberry Flavor - Rx only - 16 fl. oz.473 ...
-
Package/Label Display PanelNDC 10631-206-02 - Riomet® (metformin hydrochloride oral solution) 500 mg/5 mL - Each 5 mL contains: 500 mg of metformin hydrochloride, USP. Cherry Flavor - Rx only - 16 fl. oz.473 ...
-
INGREDIENTS AND APPEARANCEProduct Information