Label: REVONTO- dantrolene sodium injection, powder, lyophilized, for solution
- NDC Code(s): 78670-003-67
- Packager: USWM, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
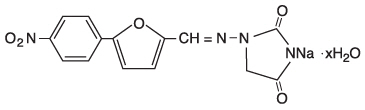
DESCRIPTIONRevonto is a sterile, non-pyrogenic, lyophilized formulation of dantrolene sodium for injection. Revonto is supplied in 65 mL vials containing 20 mg dantrolene sodium, 3000 mg mannitol, and ...
-
CLINICAL PHARMACOLOGYIn isolated nerve-muscle preparation, dantrolene sodium has been shown to produce relaxation by affecting the contractile response of the muscle at a site beyond the myoneural junction. In ...
-
INDICATIONS AND USAGERevonto (dantrolene sodium for injection) is indicated, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of ...
-
CONTRAINDICATIONSNone.
-
WARNINGSThe use of Revonto in the management of malignant hyperthermia crisis is not a substitute for previously known supportive measures. These measures must be individualized, but it will usually be ...
-
PRECAUTIONSGeneral - Care must be taken to prevent extravasation of dantrolene sodium intravenous solution into the surrounding tissues due to the high pH of the intravenous formulation and potential for ...
-
ADVERSE REACTIONSThere have been occasional reports of death following malignant hyperthermia crisis even when treated with intravenous dantrolene; incidence figures are not available (the pre-dantrolene mortality ...
-
OVERDOSAGEBecause Revonto must be administered at a low concentration in a large volume of fluid, acute toxicity of dantrolene sodium could not be assessed in animals. In 14-day (subacute) studies, the ...
-
DOSAGE AND ADMINISTRATIONAs soon as the malignant hyperthermia reaction is recognized, all anesthetic agents should be discontinued; the administration of 100% oxygen is recommended. Revonto should be administered by ...
-
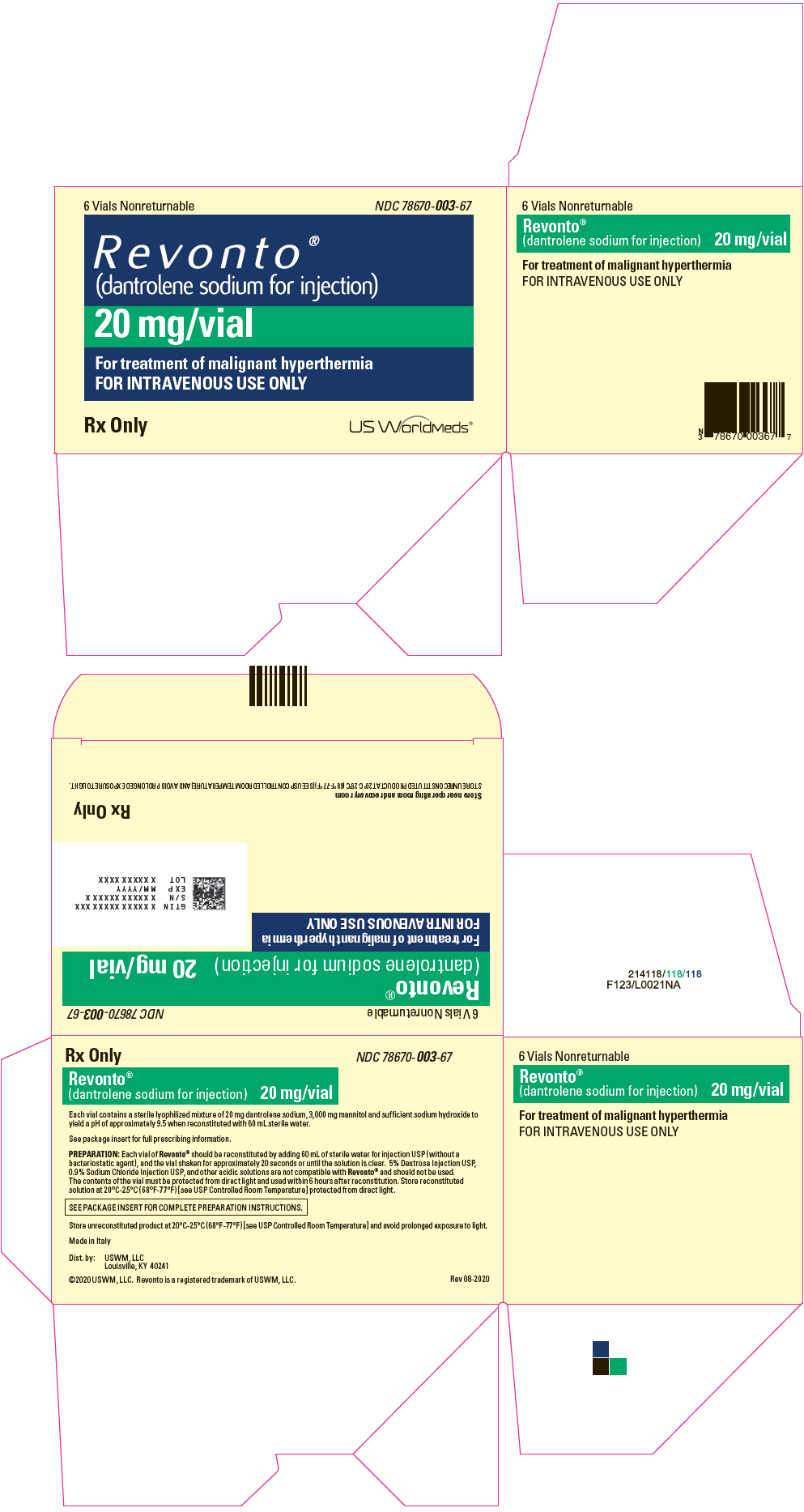
HOW SUPPLIEDRevonto (NDC 78670-003-67) is available in vials containing a sterile lyophilized mixture of 20 mg dantrolene sodium, 3000 mg mannitol, and sufficient sodium hydroxide to yield a pH of ...
-
SPL UNCLASSIFIED SECTIONRx Only - Address medical inquiries to USWM, LLC, 4441 Springdale Road, Louisville, KY 40241 - Made in Italy - Dist. by: USWM, LLC - Louisville, KY 40241 - ©2024 USWM, LLC. Revonto is a registered ...
-
PRINCIPAL DISPLAY PANEL - 20 mg Vial Carton6 Vials Nonreturnable - NDC 78670-003-67 - Revonto ® (dantrolene sodium for injection) 20 mg/vial - For treatment of malignant hyperthermia - FOR INTRAVENOUS USE ONLY - Rx Only - US WorldMeds®
-
INGREDIENTS AND APPEARANCEProduct Information