Label: YUPELRI- revefenacin solution
- NDC Code(s): 49502-806-32, 49502-806-33, 49502-806-77, 49502-806-87, view more
- Packager: Viatris Specialty LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use YUPELRI® safely and effectively. See full prescribing information for YUPELRI. YUPELRI (revefenacin) inhalation solution, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE YUPELRI is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
-
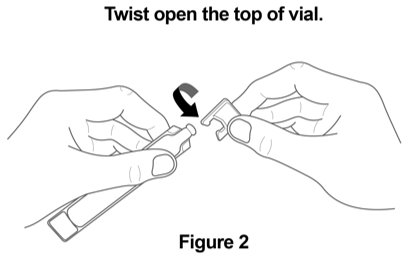
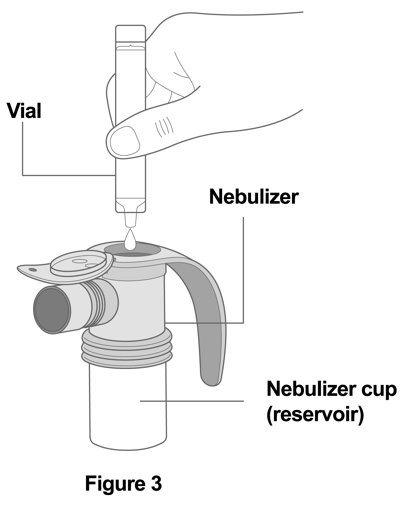
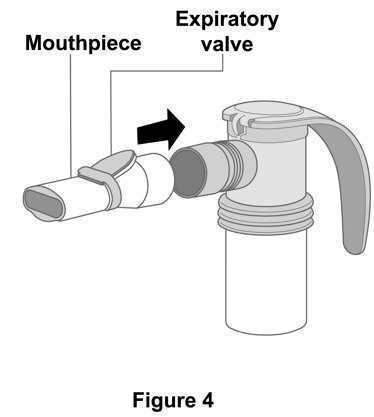
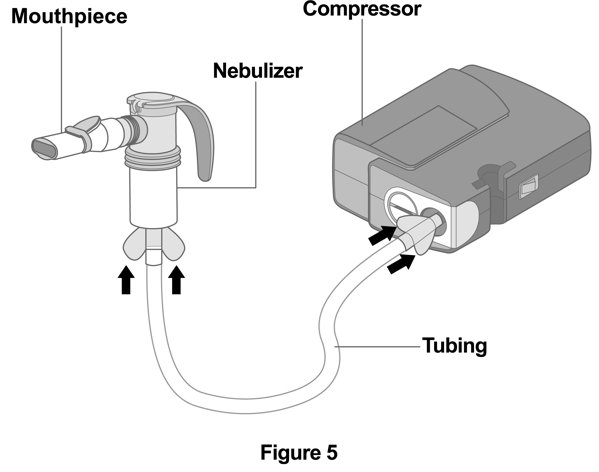
2 DOSAGE AND ADMINISTRATION The recommended dosage is 175 mcg YUPELRI (one 175 mcg unit‑dose vial) administered by oral inhalation once daily by nebulizer using a mouthpiece. Administration Overview - YUPELRI should be ...
-
3 DOSAGE FORMS AND STRENGTHS Inhalation solution: 175 mcg of revefenacin in 3 mL of sterile, clear, colorless, aqueous solution in unit dose vials.
-
4 CONTRAINDICATIONS YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product.
-
5 WARNINGS AND PRECAUTIONS 5.1 Deterioration of Disease and Acute Episodes - YUPELRI should not be initiated in patients during acutely deteriorating or potentially life-threatening episodes of COPD. YUPELRI has not been ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in labeling: • Paradoxical bronchospasm [see Warnings and Precautions (5.2)] • Worsening of narrow-angle glaucoma ...
-
7 DRUG INTERACTIONS 7.1 Anticholinergics - There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of YUPELRI with other ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with YUPELRI in pregnant women. Women should be advised to contact their physician if they become pregnant ...
-
10 OVERDOSAGE An overdosage of YUPELRI may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision ...
-
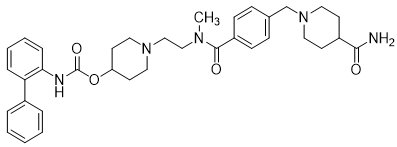
11 DESCRIPTION YUPELRI is a sterile, clear, colorless, aqueous solution of revefenacin. Revefenacin, the active component of YUPELRI, is an anticholinergic. The chemical name for revefenacin is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Revefenacin is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year inhalation studies in Sprague-Dawley rats and CD1 mice were conducted to assess the carcinogenic potential of revefenacin. No ...
-
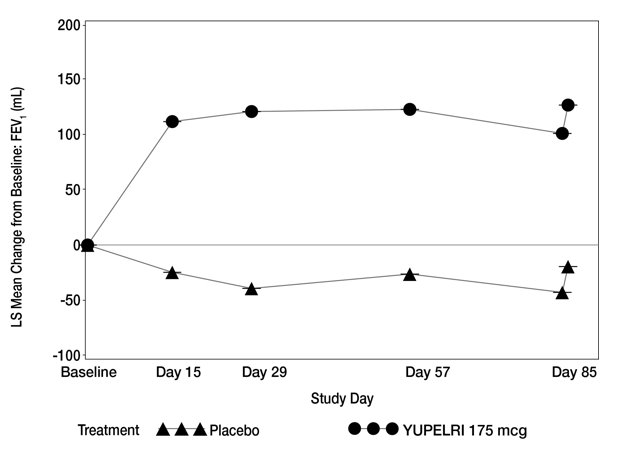
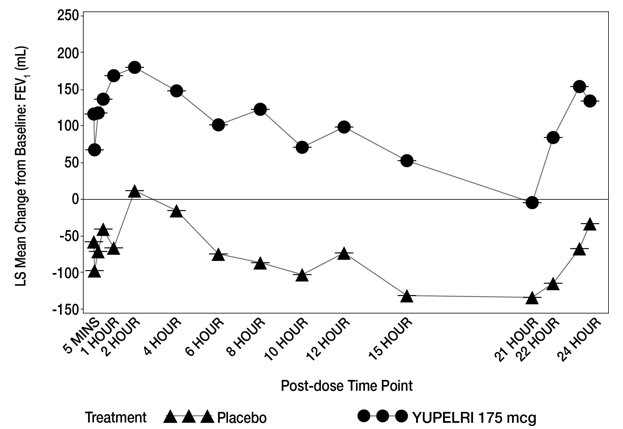
14 CLINICAL STUDIES The safety and efficacy of YUPELRI 175 mcg once daily were evaluated in two dose‑ranging trials, two replicate 12-week, Phase 3 confirmatory clinical trials, and a 52-week safety trial. The ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Inhalation solution: YUPELRI is supplied as a 175 mcg/3 mL sterile, clear, colorless, aqueous solution in unit-dose low‑density polyethylene vials. Each vial is overwrapped in a foil pouch and ...
-
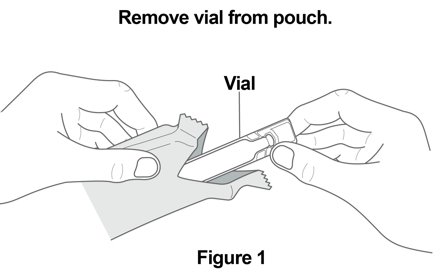
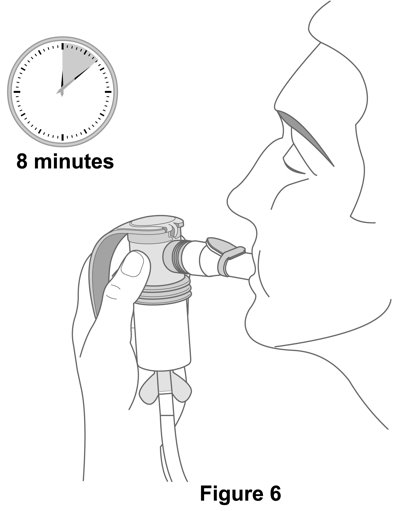
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Not for Acute Symptoms - Inform patients that YUPELRI is not meant to relieve acute ...
-
PATIENT INFORMATION YUPELRI® (you-PELL-ree) (revefenacin) inhalation solution, for oral inhalation - Important: For oral inhalation only. Do not swallow or inject YUPELRI. What is ...
-
PRINCIPAL DISPLAY PANEL – 175mcg/3 mL NDC 49502-806-93 - Rx only - YUPELRI® (revefenacin) inhalation solution - 175 mcg/3 mL - For Oral Inhalation Only - 30 sterile - unit-dose vials - Contents: Each vial contains 175 mcg of ...
-
INGREDIENTS AND APPEARANCEProduct Information