Label: RESTASIS MULTIDOSE- cyclosporine emulsion
- NDC Code(s): 0023-5301-05, 0023-5301-08
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RESTASIS MULTIDOSE safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
RESTASIS MULTIDOSE® ophthalmic emulsion is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with ...
-
2
DOSAGE AND ADMINISTRATION
Instill one drop of RESTASIS MULTIDOSE ophthalmic emulsion twice a day in each eye approximately 12 hours apart. RESTASIS MULTIDOSE can be used concomitantly with lubricant eye drops, allowing a ...
-
3
DOSAGE FORMS AND STRENGTHS
Ophthalmic emulsion containing cyclosporine 0.05% (0.5 mg/mL)
-
4
CONTRAINDICATIONS
RESTASIS MULTIDOSE is contraindicated in patients with known or suspected hypersensitivity to any of the ingredients in the formulation [see Adverse Reactions (6.2)].
-
5
WARNINGS AND PRECAUTIONS
5.1 - Potential for Eye Injury and Contamination - Be careful not to touch the bottle tip to your eye or other surfaces to avoid potential for eye injury and ...
-
6
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Potential for Eye Injury and Contamination [see Warnings and Precautions (5.1)] 6.1 - Clinical ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - Clinical administration of cyclosporine ophthalmic emulsion 0.05% is not detected systemically following topical ocular administration [see Clinical ...
-
11
DESCRIPTION
RESTASIS MULTIDOSE (cyclosporine ophthalmic emulsion) 0.05% contains a calcineurin inhibitor immunosuppressant with anti-inflammatory effects. Cyclosporine’s chemical name is ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Cyclosporine is an immunosuppressive agent when administered systemically. In patients whose tear production is presumed to be suppressed due to ocular ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Systemic carcinogenicity studies were conducted in male and female mice and rats. In the 78-week oral ...
-
14
CLINICAL STUDIES
Four multicenter, randomized, adequate and well-controlled clinical studies were performed in approximately 1,200 patients with moderate to severe keratoconjunctivitis sicca. Cyclosporine ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
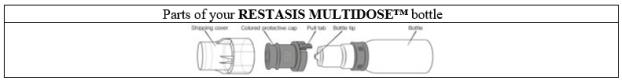
RESTASIS MULTIDOSE ophthalmic emulsion is packaged in a sterile, multi-dose bottle. Each bottle consists of a white opaque LDPE bottle, a white opaque polypropylene top with unidirectional valve ...
-
17
PATIENT COUNSELING INFORMATION
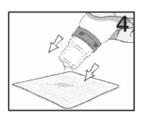
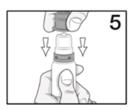
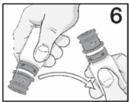
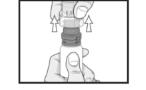
Advise the patient to read the FDA-approved patient labeling (Instructions for Use). Handling the Container - Advise patients to not allow the tip of the bottle to touch the eye or any surface ...
-
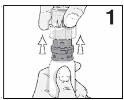
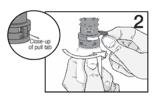
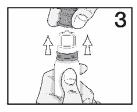
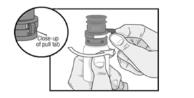
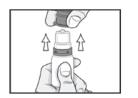
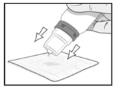
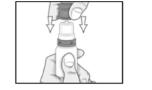
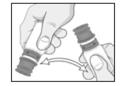
PATIENT PACKAGE INSERTINSTRUCTIONS FOR USE - RESTASIS MULTIDOSE® (Re stay’ sis Mul tee dōs) (cyclosporine ophthalmic emulsion) 0.05% Read this Instructions for Use before you start using RESTASIS MULTIDOSE and each ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL – Restasis Multidose Carton Label - NDC 0023-5301-05 - abbvie - Restasis - MultiDose™ (Cyclosporine Ophthalmic Emulsion) 0.05% For Topical Application - In the ...
-
INGREDIENTS AND APPEARANCEProduct Information


![The following structure formula for RESTASIS MULTIDOSETM is (cyclosporine ophthalmic emulsion) 0.05% contains a calcineurin inhibitor immunosuppressant with anti-inflammatory effects. Cyclosporine’s chemical name is Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl]](/dailymed/image.cfm?name=restasis-multidose-07.jpg&setid=7224d810-bb96-4682-a942-3355e6e8061a)