Label: RAYALDEE- calcifediol capsule, extended release
- NDC Code(s): 70301-1001-1, 70301-1001-2, 70301-1001-3, 70301-1002-1, view more
- Packager: OPKO Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RAYALDEE® safely and effectively. See full prescribing information for RAYALDEE. RAYALDEE® (calcifediol) extended-release ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERAYALDEE is a vitamin D3 analog indicated for the treatment of secondary hyperparathyroidism in adult patients with stage 3 or 4 chronic kidney disease and serum total 25-hydroxyvitamin D levels ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Information - • Ensure serum calcium is below 9.8 mg/dL before initiating treatment [see Warnings and Precautions (5.1)]. • Instruct patients to swallow ...
-
3 DOSAGE FORMS AND STRENGTHSExtended-release capsules, 30 mcg available as: • blue oval soft capsules imprinted with “O” in white ink - • White two-piece banded hard capsules imprinted with ”O” and “30” in blue ink
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypercalcemia - Hypercalcemia may occur during RAYALDEE treatment [see Adverse Reactions (6.1)]. Acute hypercalcemia may increase the risk of cardiac arrhythmias and seizures and may ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are discussed in greater detail in other sections of the label: • Hypercalcemia [see Warnings and Precautions (5.1)] • Adynamic Bone Disease [see ...
-
7 DRUG INTERACTIONS7.1 CYP3A Inhibitors - Cytochrome P450 inhibitors, such as ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no human data with calcifediol use in pregnant women to identify a drug-associated risk for major birth defects, miscarriage or adverse maternal or ...
-
10 OVERDOSAGEExcessive administration of RAYALDEE can cause hypercalciuria, hypercalcemia, hyperphosphatemia, or oversuppression of intact PTH. Common symptoms of vitamin D overdosage may include ...
-
11 DESCRIPTIONCalcifediol, USP, the active ingredient in RAYALDEE, is synthetically manufactured as calcifediol monohydrate. Calcifediol is also known as calcidiol, 25-hydroxycholecalciferol or ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Calcifediol (25-hydroxyvitamin D3) is a prohormone of the active form of vitamin D3, calcitriol (1,25‑dihydroxyvitamin D3). Calcifediol is converted to calcitriol by ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No neoplastic changes attributable to calcifediol were observed at subcutaneous doses of 3, 10 and 33 mcg/kg/day in a 26-week rasH2 ...
-
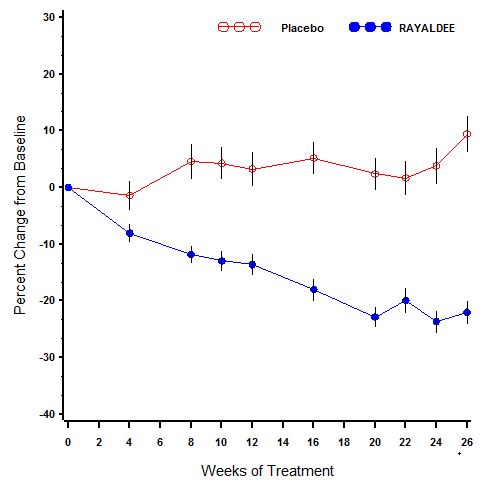
14 CLINICAL STUDIESThe efficacy and safety of RAYALDEE were evaluated in two identical multicenter, randomized, placebo-controlled, double-blind trials in patients with secondary hyperparathyroidism, stage 3 or 4 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRAYALDEE is supplied as 30 mcg calcifediol in blue, oval extended-release soft capsules, imprinted O: Bottles of 30 [NDC 70301-1001-1] Bottles of 60 [NDC 70301-1001-2] RAYALDEE is also supplied as ...
-
17 PATIENT COUNSELING INFORMATION• Inform patients to take RAYALDEE at bedtime and to swallow the capsules whole. • Inform patients if they miss a dose, to take RAYALDEE at the next scheduled time. Do not take an extra dose to ...
-
PRINCIPAL DISPLAY PANEL NDC: 70301-1001-1 - 30-count Bottle Label 30-count Soft Capsule Bottle Label - NDC 70301-1001-1 OPKO - Rayaldee® (calcifediol) Extended-Release Capsules - 30 mcg - Rx only - Each capsule contains: 30 mcg calcifediol - See ...
-
PRINCIPAL DISPLAY PANEL NDC: 70301-1002-1 - 30-count Bottle Label 30-count Hard Capsule Bottle Label - NDC 70301-1002-1 OPKO - Rx only - Rayaldee® calcifediol ER capsules - 30 mcg - Each capsule contains: 30 mcg calcifediol - See package insert ...
-
INGREDIENTS AND APPEARANCEProduct Information