Label: RAXIBACUMAB injection
- NDC Code(s): 71655-103-01
- Packager: Emergent Manufacturing Operations Baltimore LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 10, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RAXIBACUMAB safely and effectively. See full prescribing information for RAXIBACUMAB. RAXIBACUMAB injection, for intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY and ANAPHYLAXIS
- Hypersensitivity reactions, including anaphylaxis, have been reported during or after the administration of raxibacumab by intravenous infusion [see Warnings and Precautions ( 5.1)] .
- Administer raxibacumab by intravenous infusion in monitored settings where appropriate equipment, medication (including epinephrine) and personnel trained in the management of hypersensitivity, anaphylaxis, and shock are available [see Warnings and Precautions ( 2.3, 5.1)] .
-
1 INDICATIONS AND USAGE1.1 Inhalational Anthrax - Raxibacumab is indicated for the treatment of adult and pediatric patients with inhalational anthrax due to - Bacillus anthracisin combination with appropriate ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dose and Schedule for Adults - Administer raxibacumab as a single dose of 40 mg/kg intravenously over 2 hours and 15 minutes after dilution in 0.9% Sodium Chloride Injection, USP (normal ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 1,700 mg/34 mL (50 mg/mL) solution in a single-use vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity and Anaphylaxis - Hypersensitivity reactions including rash, urticaria, pruritus, chills, chest and throat tightness, lip and throat swelling, and hypotension were reported ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Ciprofloxacin - Co-administration of 40 mg/kg raxibacumab intravenously with intravenous or oral ciprofloxacin in human subjects did not alter the PK of either ciprofloxacin or raxibacumab ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on the use of raxibacumab in pregnant women to inform on drug-associated risk. In pregnant rabbits, intravenous administration of raxibacumab was ...
-
10 OVERDOSAGEThere is no clinical experience with overdosage of raxibacumab. In case of overdosage, monitor patients for any signs or symptoms of adverse effects.
-
11 DESCRIPTIONRaxibacumab is a human IgG1λ monoclonal antibody that binds the PA component of - B. anthracistoxin. Raxibacumab has a molecular weight of approximately 146 kilodaltons. Raxibacumab is produced ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Raxibacumab is a monoclonal antibody that binds the protective antigen of - B. anthracis [see Microbiology ( 12.4)]. 12.3 Pharmacokinetics - The PK of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, genotoxicity, and fertility studies have not been conducted with raxibacumab. 13.2 Animal Toxicology - Healthy ...
-
14 CLINICAL STUDIESBecause it is not feasible or ethical to conduct controlled clinical trials in humans with inhalational anthrax, the effectiveness of raxibacumab for therapeutic treatment of inhalational anthrax ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRaxibacumab is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution supplied in single-use vials containing 1,700 mg/34 mL (50 mg/mL) raxibacumab and is available ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Efficacy Based on Animal Models - Inform patients that the efficacy of raxibacumab is based solely on ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - RAXIBACUMAB (rack-see-BACK-u-mab) Injection, for intravenous use - What is the most important information I should know about RAXIBACUMAB? RAXIBACUMAB ...
-
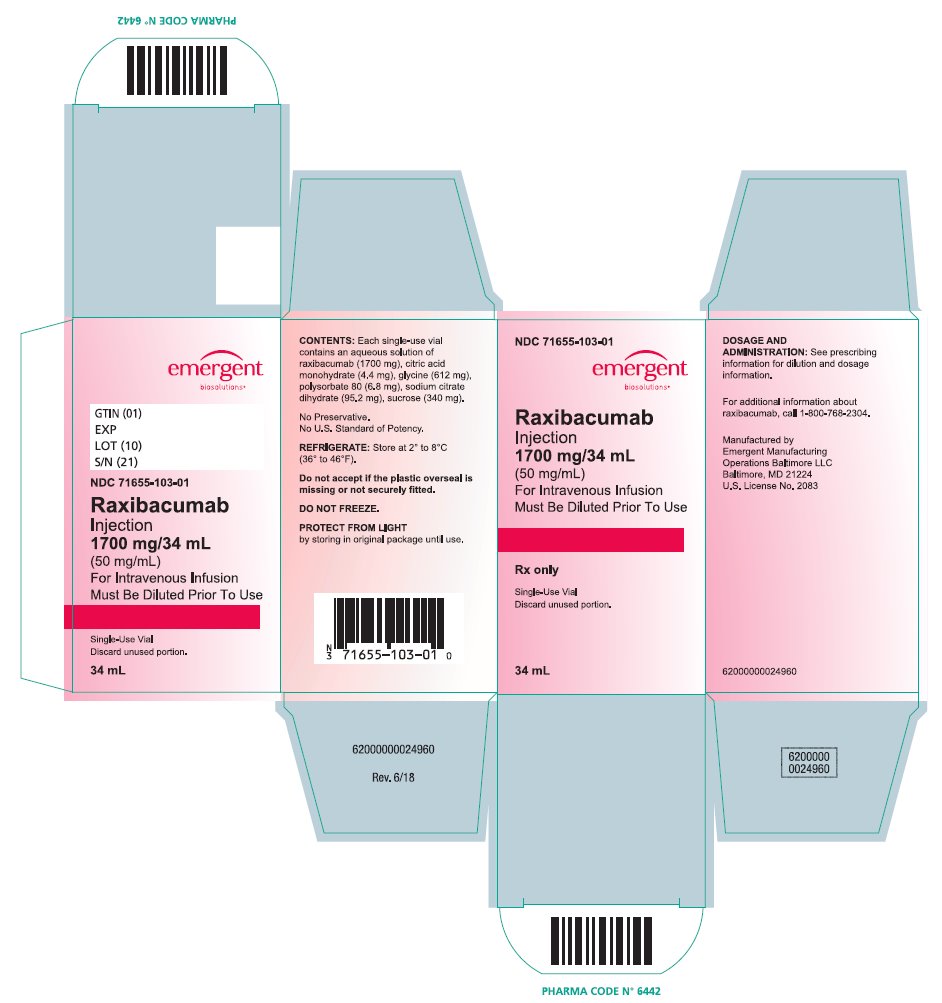
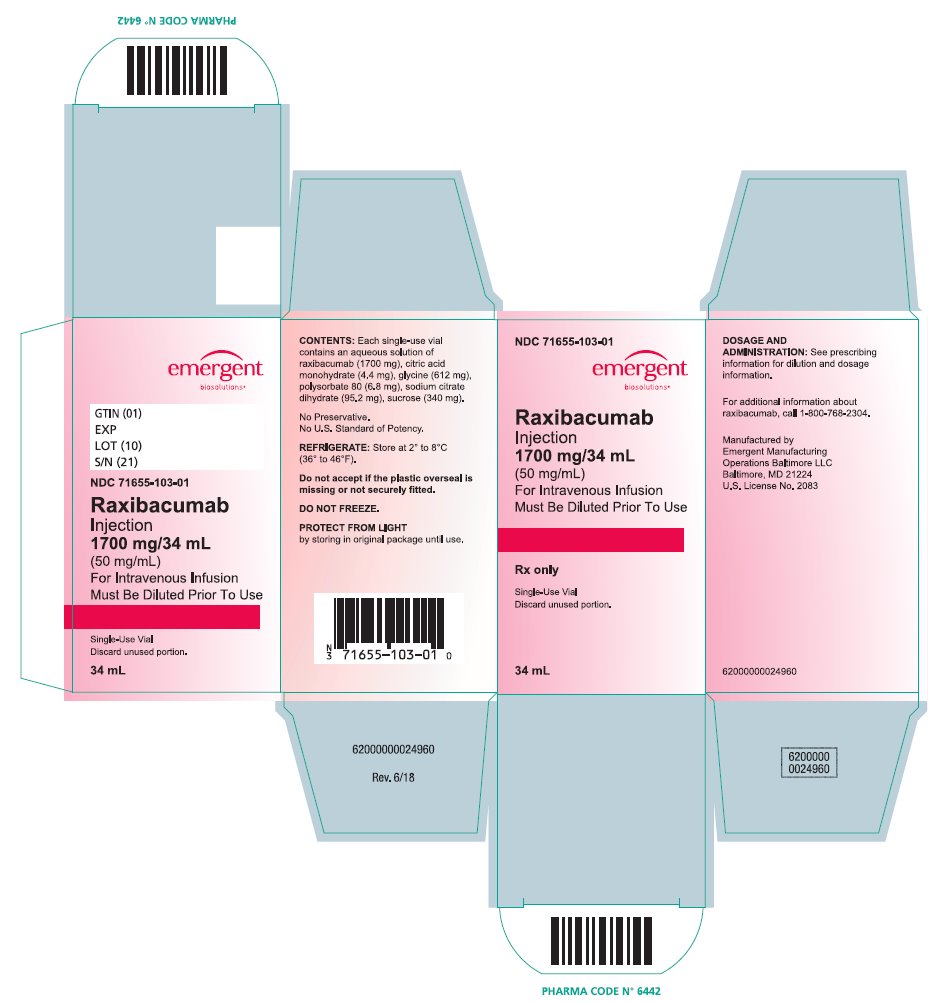
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 71655-103-01 - Raxibacumab - Injection - 1700 mg/34 mL - (50 mg/mL) For Intravenous Infusion - Must be Diluted Prior To Use - Rx only - Single-Use Vial; Discard unused ...
-
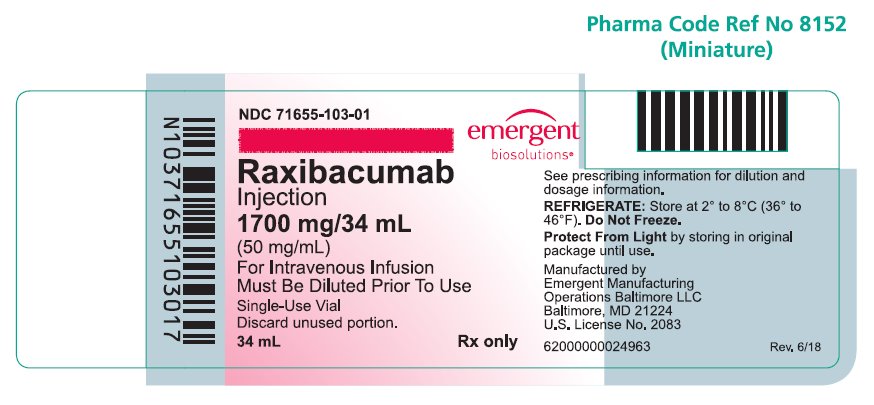
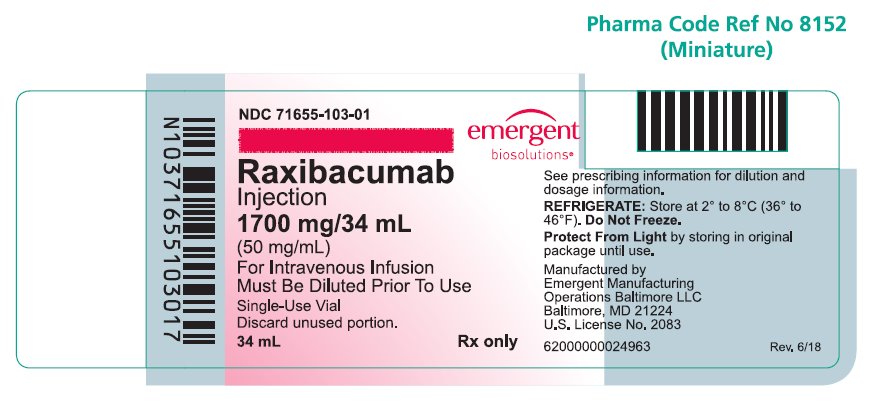
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 71655-103-01 - Raxibacumab - Injection - 1700 mg/34 mL - (50 mg/mL) For Intravenous Infusion - Must be Diluted Prior To Use - Rx only - Single-Use Vial - Discard unused ...
-
INGREDIENTS AND APPEARANCEProduct Information