Label: RADIOGARDASE- prussian blue insoluble capsules capsule
- NDC Code(s): 58060-002-02
- Packager: Heyl Chem.-pharm. Fabrik GmbH & Co. KG
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 7, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use - RADIOGARDASE safely and effectively. See full prescribing information for - RADIOGARDASE. RADIOGARDASE (prussian blue ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERadiogardase is indicated for treatment of patients with known or suspected internal contamination with radioactive cesium and/or radioactive or non-radioactive thallium, in order to increase ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Obtain quantitative baseline of the internalized contamination by radioactive cesium (137Cs) and/or thallium by appropriate whole-body counting ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 0.5 grams - dark blue capsule is imprinted with the light blue inscription: PB
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Increased Radiation Absorbed Dose to Gastrointestinal Mucosa - Radiogardase can decrease gastrointestinal motility, thus slowing the transit time of radioactivity in the gastrointestinal ...
-
6 ADVERSE REACTIONSConstipation was reported in 10 (24%) of 42 patients treated with Radiogardase. Severity of constipation was mild in 7 patients and moderate in 3 patients - [see ...
-
7 DRUG INTERACTIONSBased on animal data, co-administration of Radiogardase with other decorporation agents does not affect the efficacy of Radiogardase for treatment of internal contamination with radioactive cesium ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - It is not known whether Radiogardase can cause fetal harm when administered to a pregnant woman or if it can affect reproduction capacity ...
-
10 OVERDOSAGEBased on reported adverse reactions and mechanism of action, possible overdosage symptoms may include constipation, obstruction, or severe decrease in electrolytes. Gastric distress was reported ...
-
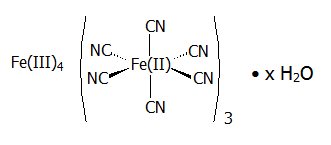
11 DESCRIPTIONRadiogardase (prussian blue insoluble) is a decorporation agent for oral use. Radiogardase capsules contain insoluble ferric hexacyanoferrate(II), with an empirical formula of Fe ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Prussian blue insoluble, ferric hexacyanoferrate(II), acts by ion-exchange, adsorption, and mechanical trapping within the crystal structure, and has a high affinity ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been performed to evaluate the carcinogenic or mutagenic potential of prussian blue insoluble. No study on ...
-
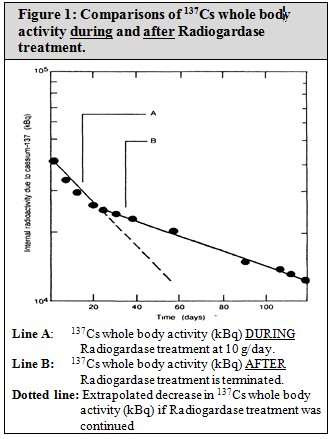
14 CLINICAL STUDIES14.1 Cesium-137 Contamination - In literature reports, 72 people received Radiogardase after exposure to radioactive cesium ( 137Cs): 46 patients with 137Cs ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRadiogardase is supplied as gelatin capsules containing 0.5 grams of prussian blue insoluble for oral administration. The dark blue capsule is imprinted with the light blue inscription ...
-
17 PATIENT COUNSELING INFORMATIONDecreased Gastrointestinal Motility - Inform patients that Radiogardase can decrease gastrointestinal motility. This can slow the transit time of cesium or thallium bound to Radiogardase and ...
-
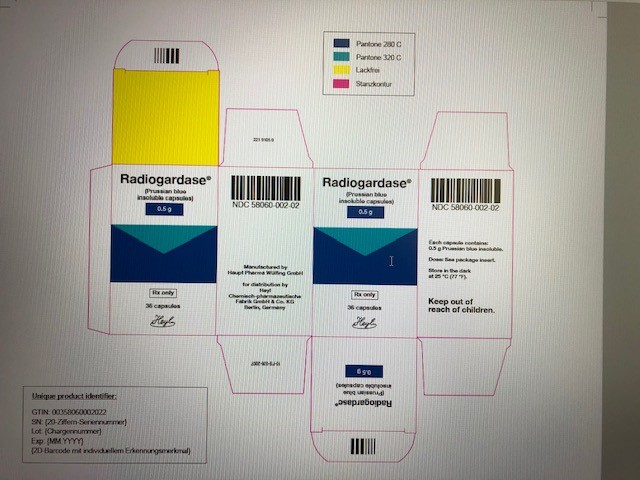
PRINCIPAL DISPLAY PANEL
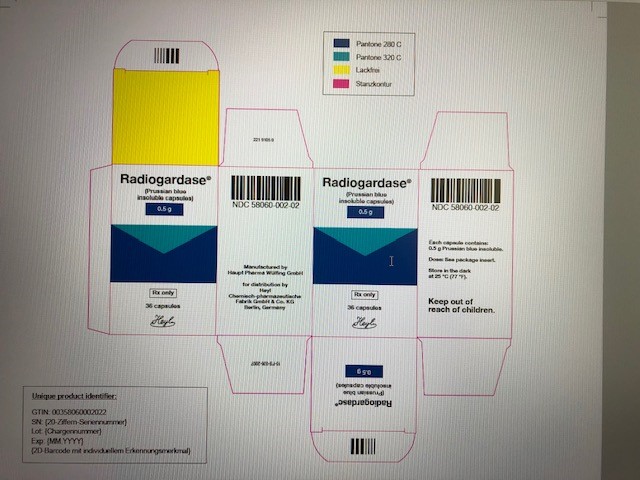 ...
... -
INGREDIENTS AND APPEARANCEProduct Information


 PB
PB
 PB. The powder may vary from uniformly fine, dark granules to coarse light and dark-colored granules. The structural formula for prussian blue insoluble is shown below.
PB. The powder may vary from uniformly fine, dark granules to coarse light and dark-colored granules. The structural formula for prussian blue insoluble is shown below.
 PB. It is packaged in white plastic containers with a child-resistant tamper-evident closure. Each container contains 36 capsules.
PB. It is packaged in white plastic containers with a child-resistant tamper-evident closure. Each container contains 36 capsules.
