Label: QBRELIS- lisinopril solution
- NDC Code(s): 52652-3001-1
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use QBRELIS - ®safely and effectively. See full prescribing information for QBRELIS. QBRELIS (lisinopril) oral solution - Initial ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE1.1 Hypertension - QBRELIS is indicated for the treatment of hypertension in adult patients and pediatric patients 6 years of age and older to lower blood pressure. Lowering blood pressure lowers ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - Adults - Initial Therapy in adults: The recommended initial dose is 10 mg taken orally once a day. Adjust dosage as needed according to blood pressure response. The usual ...
-
3 DOSAGE FORMS AND STRENGTHSQBRELIS oral solution is available in a 150 mL bottle containing 1 mg/mL of lisinopril solution. QBRELIS oral solution is a clear to slightly opalescent liquid.
-
4 CONTRAINDICATIONSQBRELIS is contraindicated in patients with: a history of angioedema or hypersensitivity related to previous treatment with an angiotensin converting enzyme inhibitor - hereditary or idiopathic ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Diuretics - Initiation of QBRELIS in patients on diuretics may result in excessive reduction of blood pressure. The possibility of hypotensive effects with QBRELIS can be minimized by either ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - QBRELIS can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters ...
-
10 OVERDOSAGEFollowing a single oral dose of 20 g/kg no lethality occurred in rats, and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be ...
-
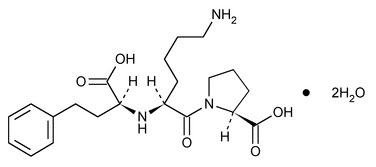
11 DESCRIPTIONLisinopril is an oral long-acting angiotensin converting enzyme (ACE) inhibitor. Lisinopril is a synthetic peptide derivative that is manufactured as a dihydrate and is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lisinopril inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 ...
-
14 CLINICAL STUDIES14.1 Hypertension - Two dose-response studies utilizing a once-daily regimen were conducted in 438 mild to moderate hypertensive patients not on a diuretic. Blood pressure was measured 24 hours ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGQBRELIS (lisinopril), 1 mg/mL, is supplied as 150 mL of a clear to slightly opalescent, colorless aqueous oral solution with a sweet taste in a 150-mL high-density polyethylene (HDPE) bottle with ...
-
17 PATIENT COUNSELING INFORMATIONPregnancy: Tell female patients of childbearing age about the consequences of exposure to QBRELIS during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients ...
-
Manufactured for: Azurity Pharmaceuticals, Inc. Woburn, MA 01801 USA - Patent: https://azurity.com/patents - This product’s label may have been updated. For current Full Prescribing ...
-
PRINCIPAL DISPLAY PANEL - Bottle LabelNDC 52652-3001-1 - Qbrelis - ® (lisinopril) Oral Solution - 1 mg/mL - READY TO USE - 150 mL - azurity™ pharmaceuticals - Rx Only - Each 1 mL contains - 1 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information