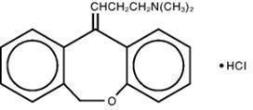
General Drowsiness: Since drowsiness may occur with the use of PRUDOXIN® Cream, patients should be warned of the possibility and cautioned against driving a car or operating dangerous machinery ...
General Drowsiness: Since drowsiness may occur with the use of PRUDOXIN® Cream, patients should be warned of the possibility and cautioned against driving a car or operating dangerous machinery while using this drug. Patients should also be cautioned that their response to alcohol may be potentiated.
Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on PRUDOXIN® Cream. (See PRECAUTIONS - Geriatric Use.)
Use under occlusion: Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with PRUDOXIN® Cream.
Contact sensitization: Use of PRUDOXIN® Cream can cause Type IV hypersensitivity reactions (contact sensitization) to doxepin.
Drug Interactions
Studies have not been performed examining drug interactions with PRUDOXIN® Cream. However, since plasma levels of doxepin following topical application of PRUDOXIN® Cream can reach levels obtained with oral doxepin HCl therapy, the following drug interactions are possible following topical PRUDOXIN® Cream application:
Drugs Metabolized by P450 2D6: The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7-10% of Caucasians are so-called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available.
Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dosage regimen of a TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half- life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6.
MAO Inhibitors: Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Therefore, MAO inhibitors should be discontinued at least two weeks prior to the cautious initiation of therapy with PRUDOXIN® Cream. The exact length of time may vary and is dependent upon the particular MAO inhibitor being used, the length of time it has been administered, and the dosage involved.
Cimetidine: Serious anticholinergic symptoms (i.e., severe dry mouth, urinary retention and blurred vision) have been associated with elevations in the serum levels of tricyclic antidepressant when cimetidine therapy is initiated. Additionally, higher than expected tricyclic antidepressant levels have been observed when they are begun in patients already taking cimetidine.
Alcohol: Alcohol ingestion may exacerbate the potential sedative effects of PRUDOXIN® Cream. This is especially important in patients who may use alcohol excessively.
Tolazamide: A case of severe hypoglycemia has been reported in a type II diabetic patient maintained on tolazamide (1 gm/day) 11 days after the addition of oral doxepin (75 mg/day).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, mutagenesis, and impairment of fertility studies have not been conducted with doxepin hydrochloride.
Pregnancy
Reproduction studies have been performed in which doxepin was orally administered to rats and rabbits at doses up to 0.6 and 1.2 times, respectively, the estimated exposure to doxepin that results from use of 16 grams of PRUDOXIN® Cream per day (four applications of four grams of cream per day; dose multiples reflect comparisons made following normalization of the data on the basis of body surface area estimates) and have revealed no evidence of harm to rat or rabbit fetuses due to doxepin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Doxepin is excreted in human milk after oral administration. It is possible that doxepin may also be excreted in human milk following topical application of PRUDOXIN® Cream.
One case has been reported of apnea and drowsiness in a nursing infant whose mother was taking an oral dosage form of doxepin HCl.
Because of the potential for serious adverse reactions in nursing infants from doxepin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The use of PRUDOXIN® Cream in pediatric patients is not recommended. Safe conditions for use of PRUDOXIN® Cream in children have not been established. One case has been reported of a 2.5 year old child who developed somnolence, grand mal seizure, respiratory depression, ECG abnormalities, and coma after treatment with PRUDOXIN® Cream. A total of 27 grams had been applied over three days for eczema. He was treated with supportive care, activated charcoal, and systemic alkalization and recovered.
Geriatric Use
Clinical studies of PRUDOXIN® Cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
The extent of renal excretion of doxepin has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be observed closely for confusion and oversedation when started on PRUDOXIN® Cream. (See WARNINGS.) An 80-year old male nursing home patient developed probable systemic anticholinergic toxicity which included urinary retention and delirium after PRUDOXIN® Cream had been applied to his arms, legs and back three times daily for two days.
Close