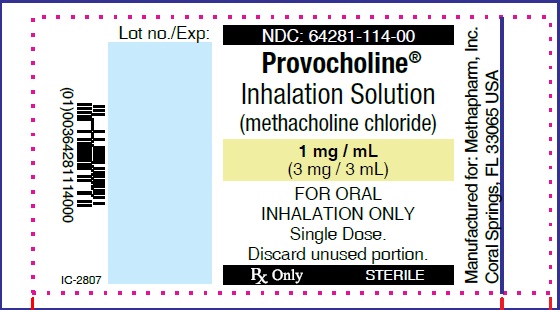
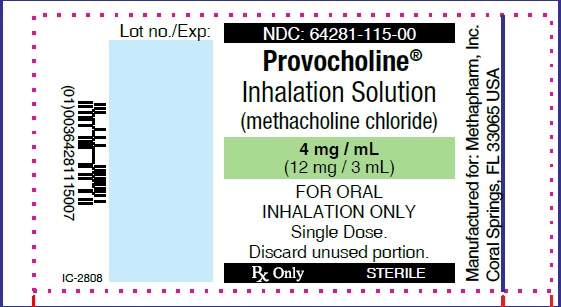
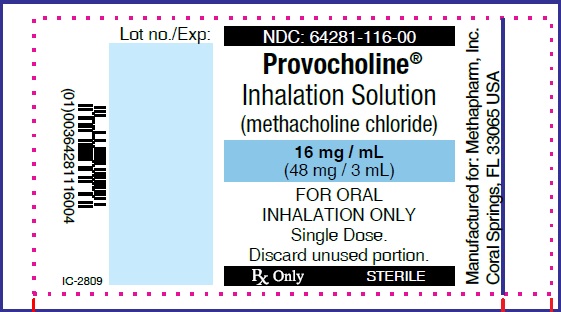
Label: PROVOCHOLINE- methacholine chloride powder, for solution
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...view full title
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...
- NDC Code(s): 64281-100-00, 64281-100-06, 64281-110-05, 64281-110-06, view more
- Packager: Methapharm Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PROVOCHOLINE® safely and effectively. See full prescribing information for PROVOCHOLINE®. PROVOCHOLINE® (methacholine chloride ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEVERE BRONCHOCONSTRICTION
WARNING: SEVERE BRONCHOCONSTRICTION
Severe bronchoconstriction can result from Provocholine administration (including the lowest dose). The use of Provocholine is contraindicated in pediatric and adult patients with baseline FEV1 < 60% predicted or adults with FEV1 < 1.5 L. Because of the potential for severe bronchoconstriction, the use of Provocholine in patients with clinically apparent asthma or wheezing is not recommended [see Warnings and Precautions (5.1)].
Emergency equipment and medication should be immediately available to treat acute respiratory distress. If severe bronchoconstriction occurs, reverse immediately with a rapid-acting inhaled bronchodilator agent (β-agonist) [see Warnings and Precautions (5.1)].
If baseline spirometry is not performed or is measured inaccurately, the initial FEV1 may be underestimated. In this situation, decreases in FEV1 may not be detected after administration of escalating Provocholine doses, which may result in administration of unnecessary higher doses and an increased risk for excessive bronchoconstriction [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGE
Provocholine, used in a methacholine challenge test, is indicated for the diagnosis of bronchial airway hyperreactivity in adults and pediatric patients five years of age and older who do not have ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Methacholine Challenge Test Overview - Provocholine should be administered in a methacholine challenge test in a pulmonary function laboratory or clinic, by adequately trained personnel, for ...
-
2.2 Recommended DosageThe recommended dosage of Provocholine powder for inhalation solution (require reconstitution and dilution) or inhalation solution (in a ready-to-use kit) used in the methacholine challenge test ...
-
2.3 Reconstitution and Dilution Prior to Administration1. Provocholine Powder for Inhalation Solution requires reconstitution before use (see Tables 1 and 2): Add 6.25 mL of 0.9% Sodium Chloride Injection (0.9% saline) or 0.9% Sodium Chloride ...
-
2.4 Administration with the Five (5)-Breath Dosimeter Dosing Method in Patients 5 Years of Age and Older
Prior to administering the Provocholine dose(s), determine the post-diluent FEV1 value required for the methacholine challenge test. Administration of the Diluent or Base to Obtain Post-Diluent ...
-
2.5 Administration with the Two (2)-Minute Tidal Breathing Dosing Method in Patients 5 Years of Age and Older
Administer the diluent and the Provocholine dose(s) using the English Wright nebulizer or other suitable nebulizer as long as the device output and particle size are characterized. Prior to ...
-
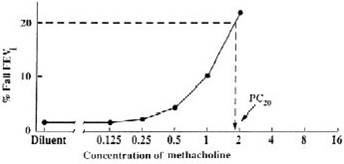
2.6 Calculation and Interpretation of Methacholine Challenge Test ResultsA positive methacholine challenge test is a ≥ 20% reduction in the FEV1 (after Provocholine oral inhalation) compared with the mean post-diluent FEV1. Calculate and record post-diluent FEV1 value ...
-
3 DOSAGE FORMS AND STRENGTHS
• For inhalation solution: 100 mg of white to off-white crystalline powder in amber glass vials (powder is reconstituted and then diluted prior to administration) • Inhalation solution: o base ...
-
4 CONTRAINDICATIONS
Provocholine is contraindicated in the following: Hypersensitivity to methacholine or other parasympathomimetic agents. Reactions have included rash, itching/swelling (especially of the ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Severe Bronchoconstriction - Severe bronchoconstriction can result from Provocholine administration (including the lowest dose). The use of Provocholine is contraindicated in ...
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of Provocholine were identified in clinical studies or post marketing reports. Because some of these reactions were reported voluntarily ...
-
7 DRUG INTERACTIONS
Beta-Adrenergic Blockers - The use of beta-adrenergic blockers may impair reversal of Provocholine-caused bronchoconstriction. Beta-Agonists, Anticholinergics, and Theophylline - Beta-agonists ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - The available data from published literature on Provocholine use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects ...
-
11 DESCRIPTION
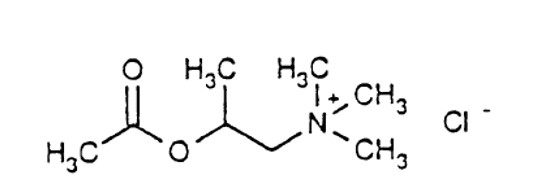
Methacholine chloride, the active ingredient of Provocholine, is a parasympathomimetic (cholinergic) bronchoconstrictor agent. Provocholine (methacholine chloride) powder for solution is ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Methacholine chloride is a cholinergic agonist. Bronchial smooth muscle contains significant parasympathetic (cholinergic) innervation. Methacholine chloride ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There have been no studies with methacholine chloride that would permit an evaluation of its carcinogenic or mutagenic potential or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - Provocholine (methacholine chloride) Powder for Inhalation Solution: in amber glass vials that contain 100 mg of methacholine chloride powder, white to off-white in color. Cartons ...
-
17 PATIENT COUNSELING INFORMATIONRisk of Severe Bronchoconstriction - Inform the patient or caregiver that severe bronchoconstriction can result from Provocholine administration [see Warnings and Precautions (5.1)]. Cough, Chest ...
-
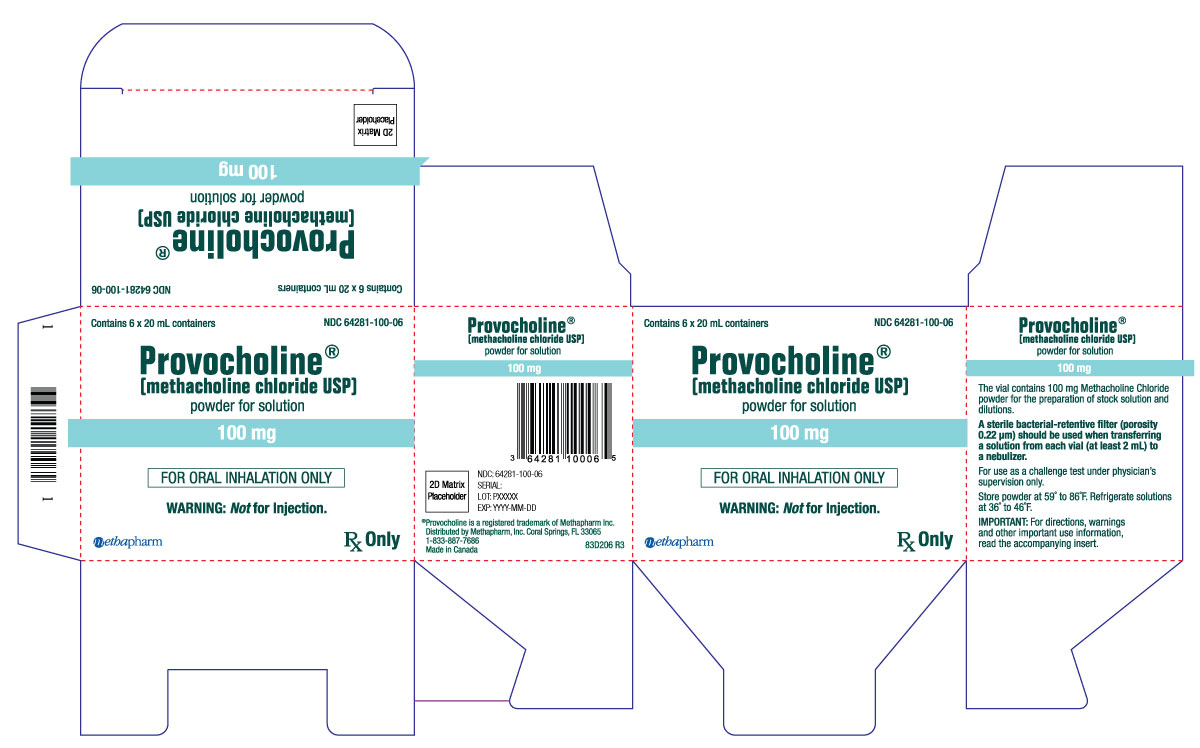
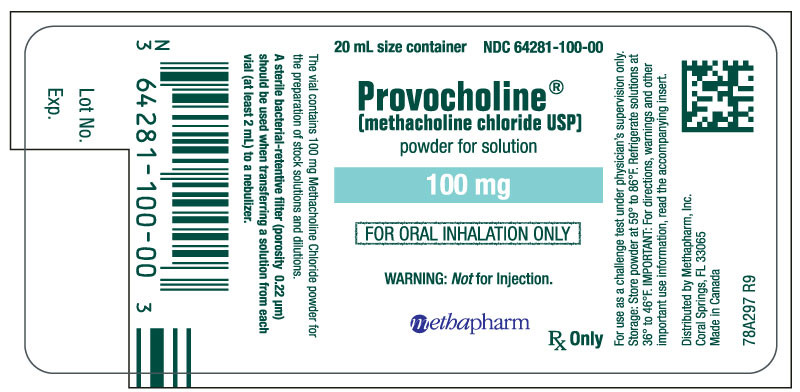
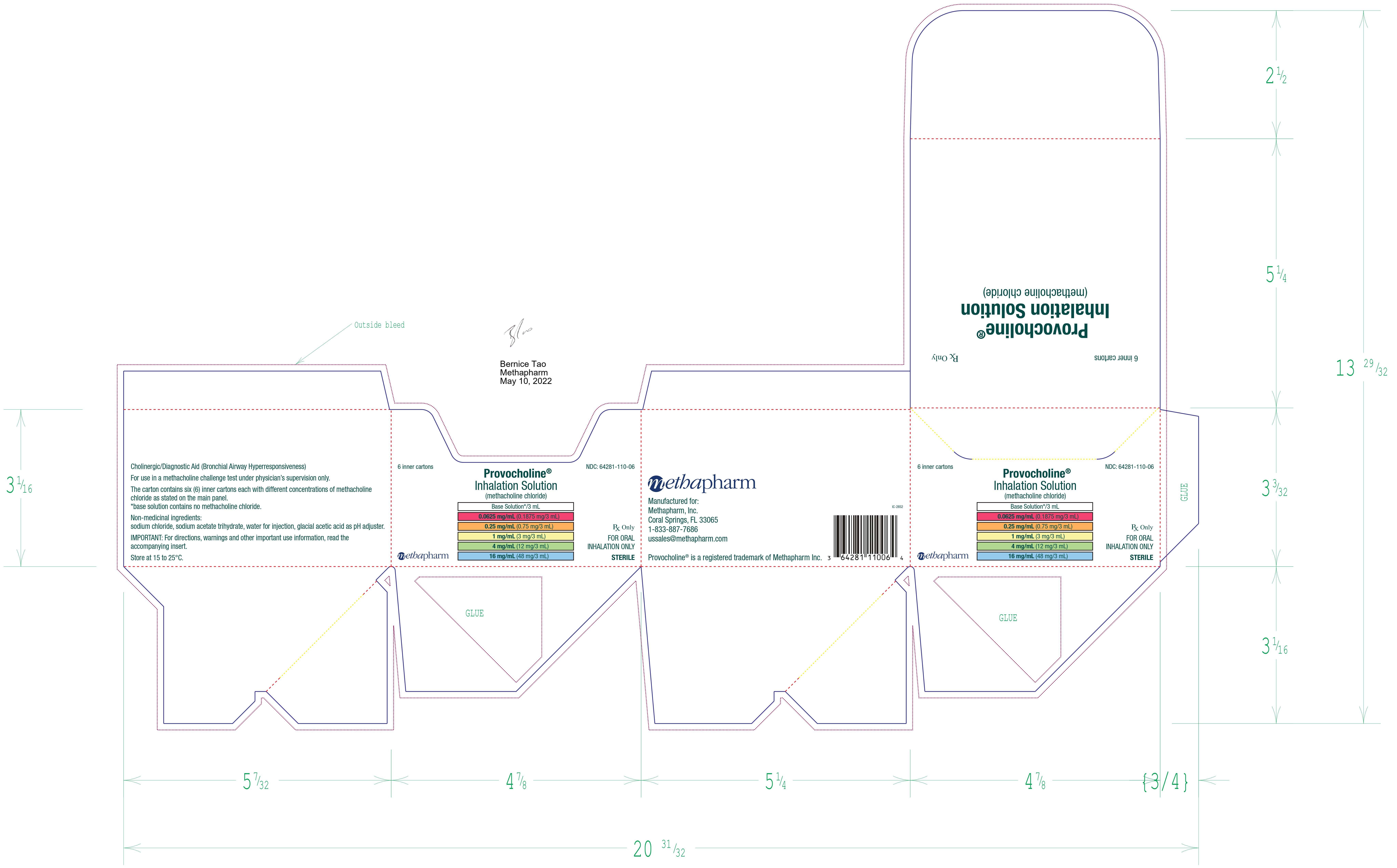
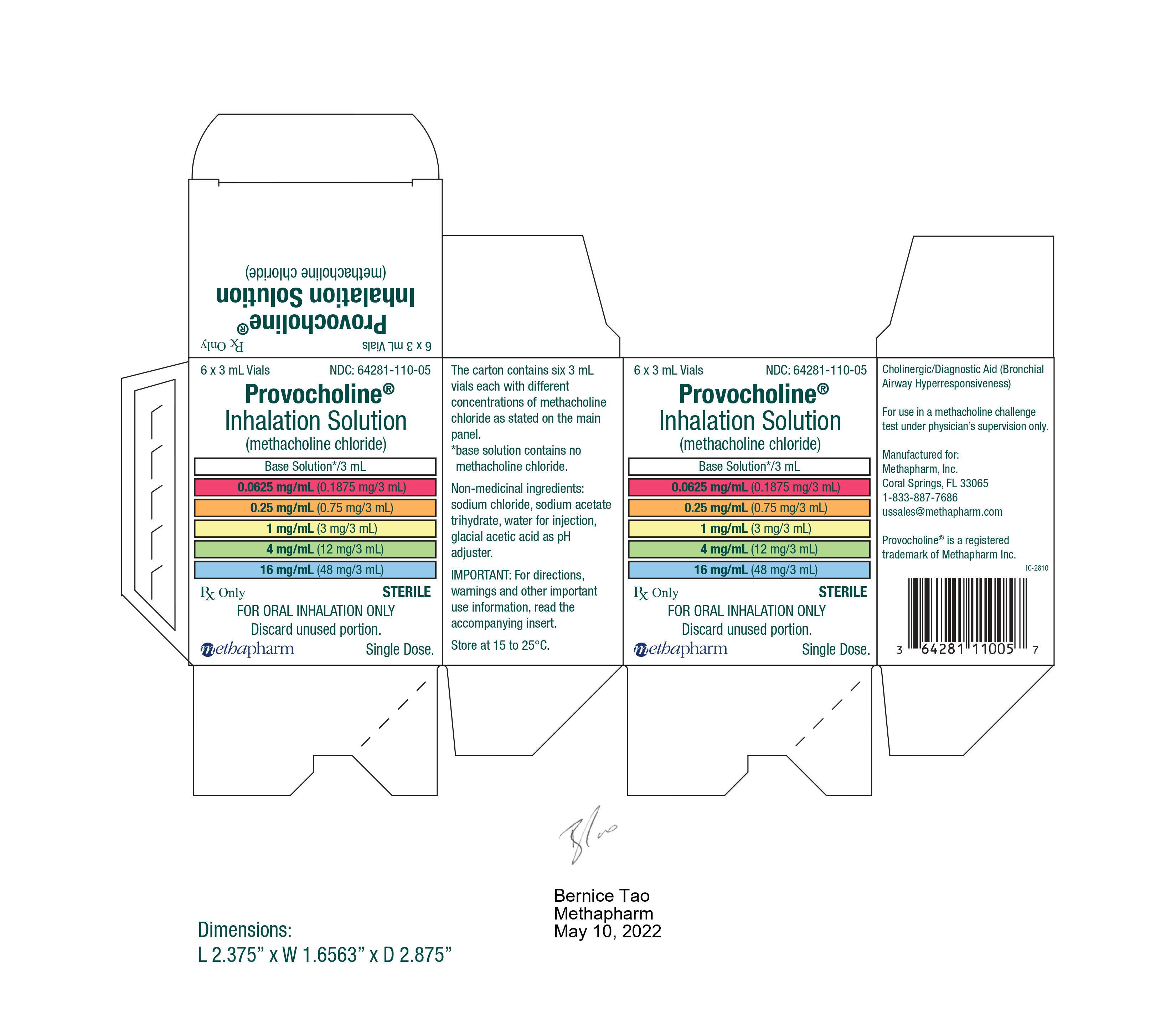
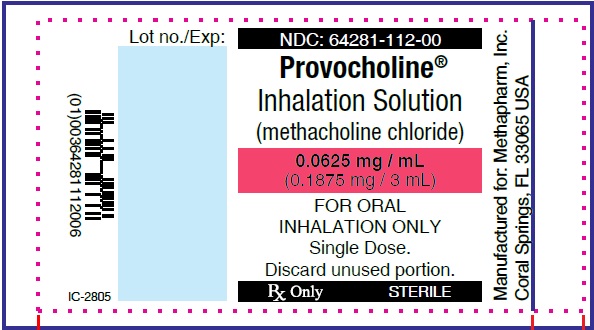
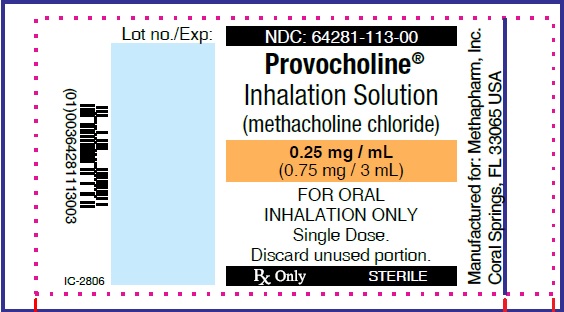
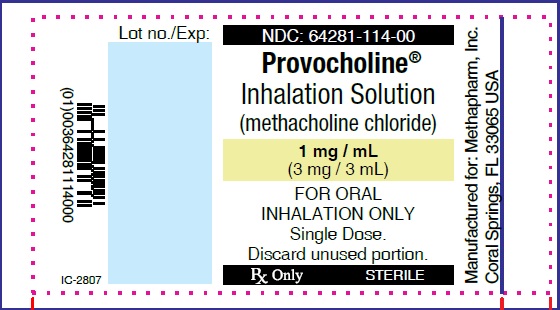
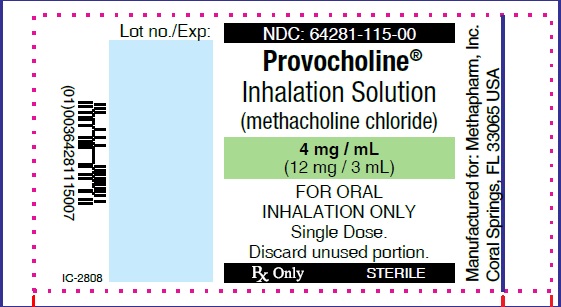
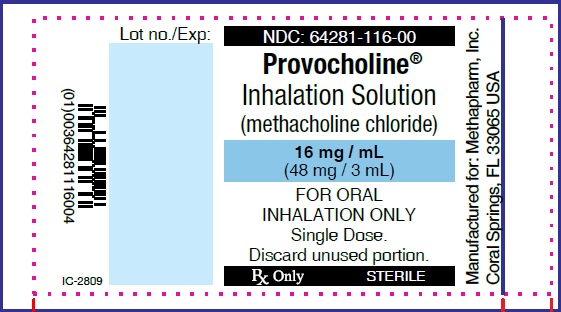
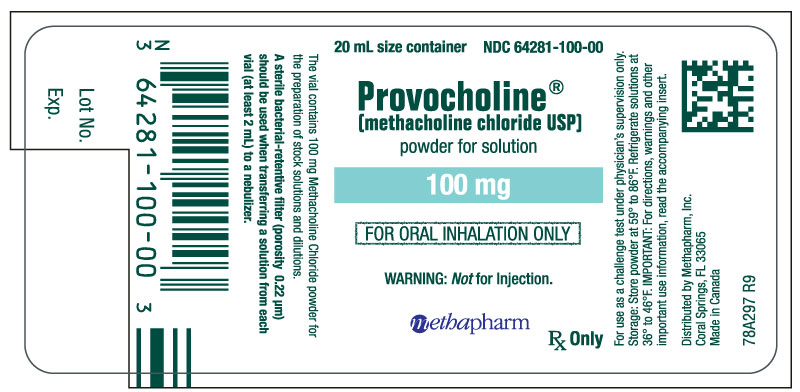
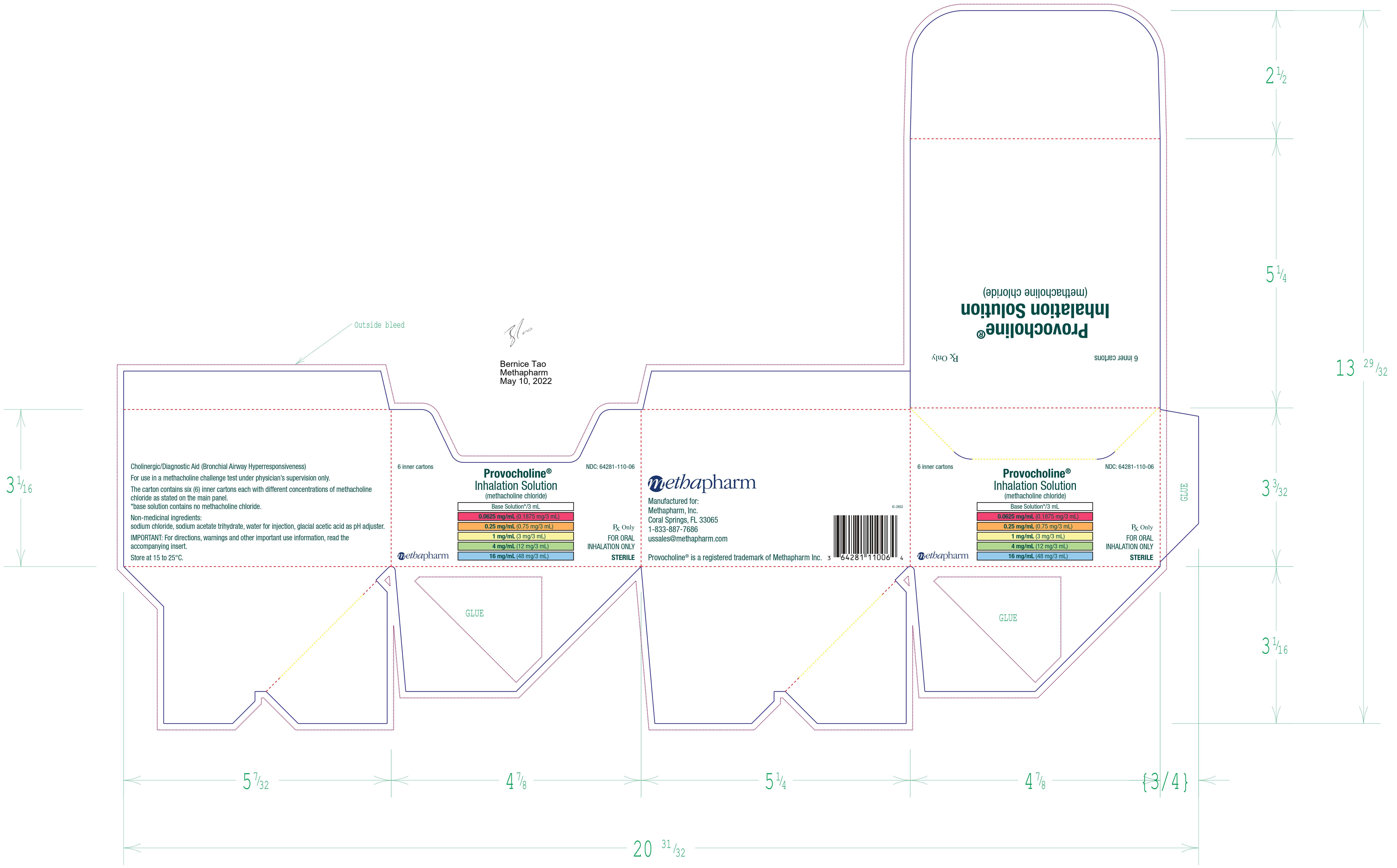
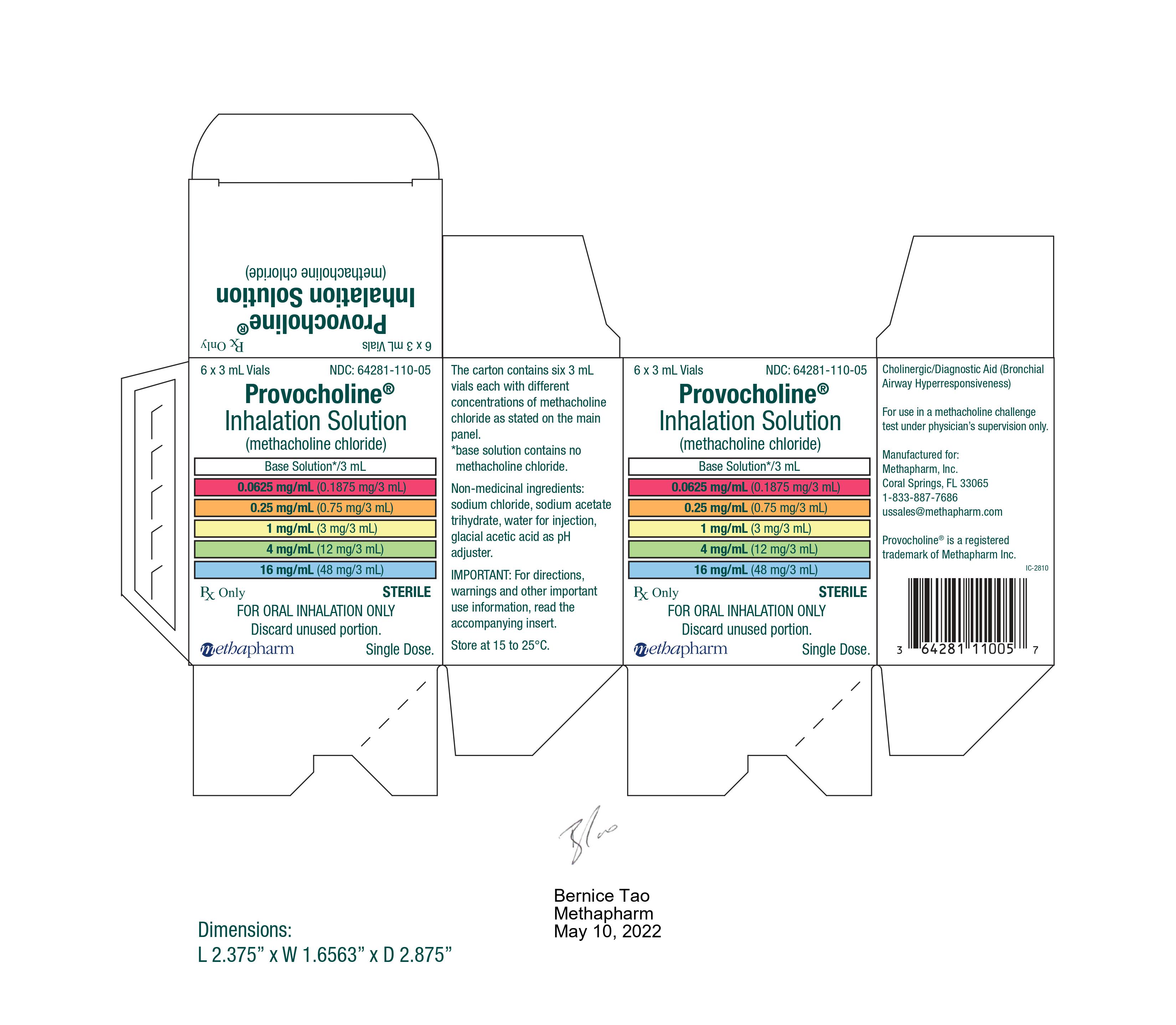
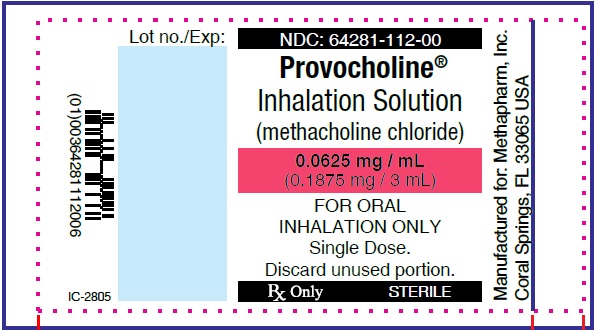
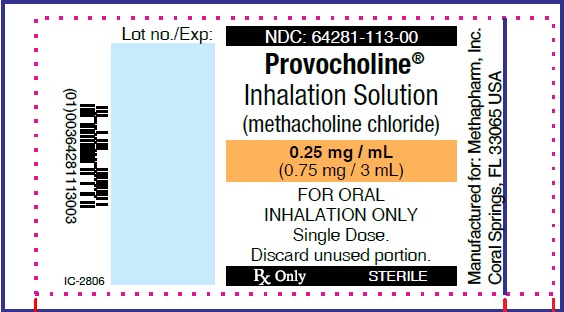
PRINCIPAL DISPLAY PANEL
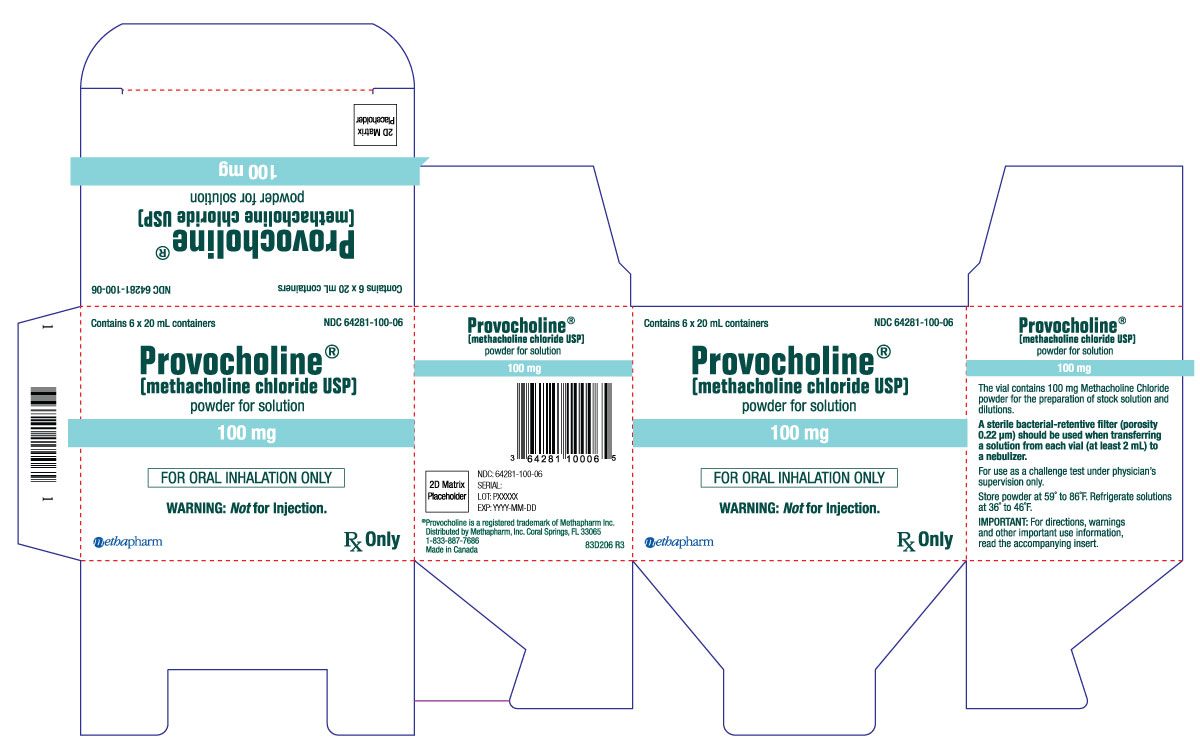 ...
... -
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
PROVOCHOLINE- methacholine chloride powder, for solution
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...view full title
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...
Number of versions: 19
RxNorm
PROVOCHOLINE- methacholine chloride powder, for solution
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...view full title
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...
Get Label RSS Feed for this Drug
PROVOCHOLINE- methacholine chloride powder, for solution
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...view full title
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...
NDC Codes
PROVOCHOLINE- methacholine chloride powder, for solution
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...view full title
PROVOCHOLINE INHALATION SOLUTION- methacholine chloride inhalati...