Label: PROTOPAM CHLORIDE- pralidoxime chloride injection, powder, lyophilized, for solution
- NDC Code(s): 60977-141-01, 60977-141-27
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
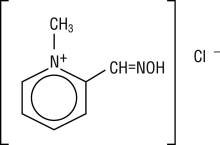
DESCRIPTIONChemical name: 2-formyl-1-methylpyridinium chloride oxime. Available in the United States as PROTOPAM Chloride for Injection (PROTOPAM Chloride), pralidoxime chloride is frequently referred to as ...
-
CLINICAL PHARMACOLOGYThe principal action of pralidoxime chloride is to reactivate cholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate ...
-
CLINICAL STUDIESThere are no adequate and well controlled clinical studies that establish the effectiveness of pralidoxime chloride as a treatment for poisoning with organophosphates having anticholinesterase ...
-
INDICATIONS AND USAGEPROTOPAM Chloride is indicated as an antidote: 1. In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have ...
-
CONTRAINDICATIONSThere are no known absolute contraindications for the use of PROTOPAM Chloride (see PRECAUTIONS, Drug Interactions and DOSAGE AND ADMINISTRATION). Relative contraindications include known ...
-
WARNINGSPROTOPAM Chloride is not effective in the treatment of poisoning due to phosphorus, inorganic phosphates, or organophosphates not having anticholinesterase activity. PROTOPAM Chloride is not ...
-
PRECAUTIONSGeneral - PROTOPAM Chloride has been well tolerated in most cases, but it must be remembered that the desperate condition of the organophosphate-poisoned patient will generally mask such minor ...
-
ADVERSE REACTIONSForty to 60 minutes after intramuscular injection, mild to moderate pain may be experienced at the site of injection. Pralidoxime chloride may cause blurred vision, diplopia and impaired ...
-
DRUG ABUSE AND DEPENDENCEPROTOPAM Chloride is not subject to abuse and possesses no known potential for dependence.
-
OVERDOSAGEManifestations of Overdosage - Observed in normal subjects only: dizziness, blurred vision, diplopia, headache, impaired accommodation, nausea, slight tachycardia. In therapy, it has been ...
-
DOSAGE AND ADMINISTRATIONOrganophosphate Poisoning - Treatment should include general supportive care, atropinization, and decontamination, in addition to the use of PROTOPAM Chloride. Treatment is most effective if ...
-
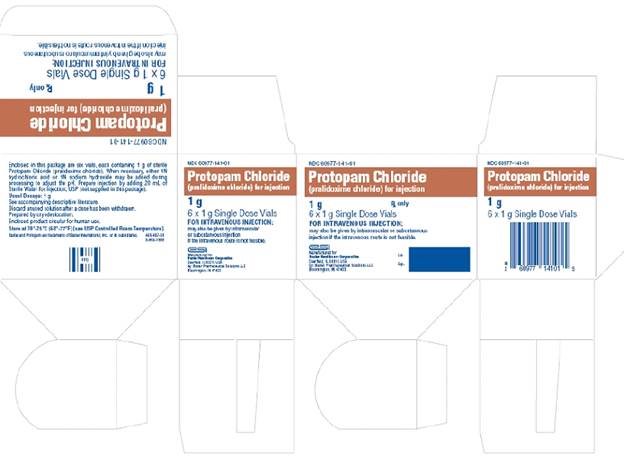
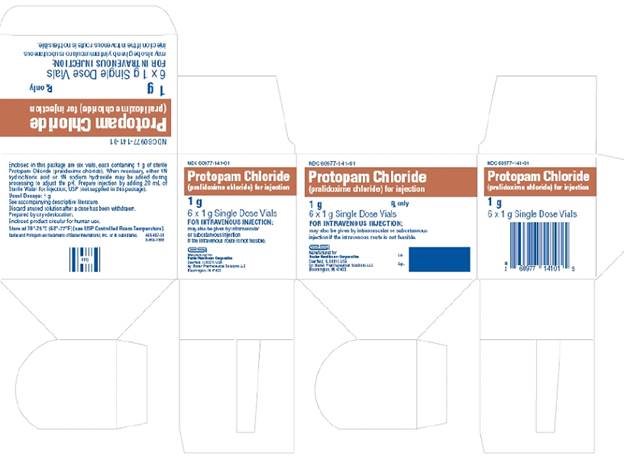
HOW SUPPLIEDNDC 60977-141-01—Hospital Package: This contains six 20 mL vials of 1 g each of sterile PROTOPAM Chloride (pralidoxime chloride) for Injection white to off-white porous cake*, without diluent or ...
-
ANIMAL PHARMACOLOGY AND TOXICOLOGYThe following table lists chemical and trade or generic names of pesticides, chemicals, and drugs against which PROTOPAM Chloride (usually administered in conjunction with atropine) has been found ...
-
SPL UNCLASSIFIED SECTIONBaxter and Protopam are trademarks of Baxter International Inc. All other trademarks or brand names appearing herein are the property of their respective owners. Manufactured for - Baxter ...
-
PACKAGE LABEL - Principal Display PanelContainer Label - Container Label - NDC 60977-141-27 - Protopam Chloride - (pralidoxime chloride) for injection - 1 g Single Dose Vial - Rx only - FOR INTRAVENOUS INJECTION; may be given ...
-
INGREDIENTS AND APPEARANCEProduct Information