Label: PROTHERIX- triamcinolone acetonide injection, suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 70529-048-01, 70529-048-02, 70529-048-03 - Packager: IT3 Medical LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONTriamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR ...
-
CLINICAL PHARMACOLOGYGlucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and ...
-
INDICATIONS AND USAGEIntramuscular - Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated for intramuscular use as follows ...
-
CONTRAINDICATIONSTriamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see WARNINGS: General). Intramuscular corticosteroid ...
-
WARNINGSSerious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see WARNINGS ...
-
PRECAUTIONSGeneral - This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial. The lowest ...
-
ADVERSE REACTIONS(listed alphabetically under each subsection) The following adverse reactions may be associated with corticosteroid therapy: Allergic reactions: Anaphylaxis including death, angioedema ...
-
OVERDOSAGETreatment of acute overdosage is by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of the corticosteroid ...
-
DOSAGE AND ADMINISTRATIONGeneral - NOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS). The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the specific ...
-
HOW SUPPLIEDTriamcinolone Acetonide Injectable Suspension, USP is supplied in vials providing 40 mg triamcinolone acetonide per mL. 40 mg/mL, 1 mL - 1 mL single-dose vial ...
-
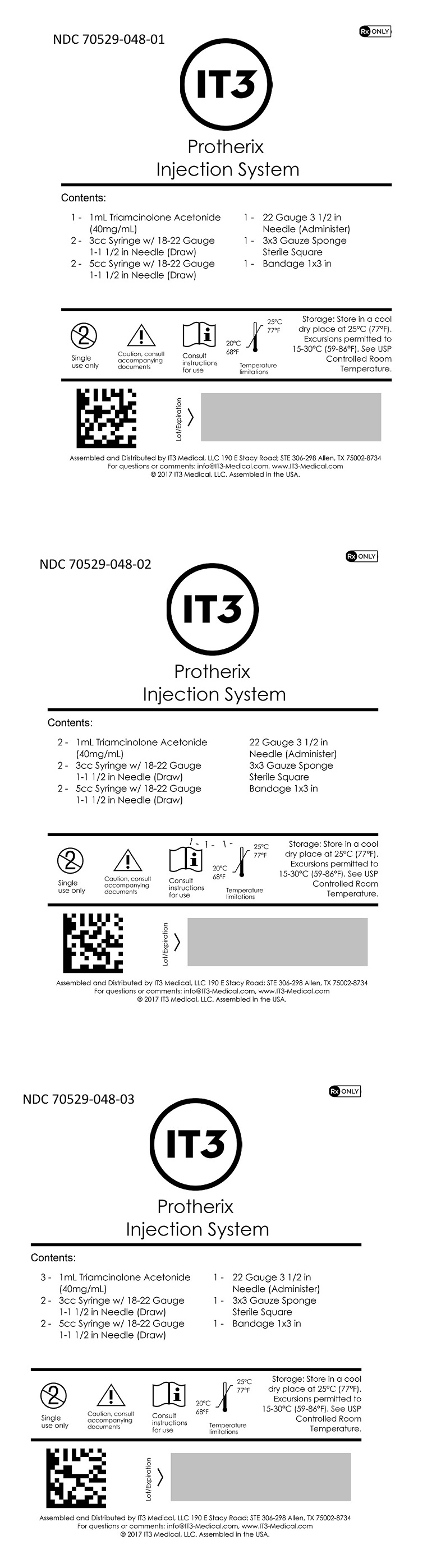
Protherix Injection SystemContents (NDC 70529-048-01): 1 - 1mL Triamcinolone Acetonide (40mg/mL) 2 - 3cc Syringe w/ 18-22 Gauge 1-1 1/2 in Needle (Draw) 2 - 5cc Syringe w/ 18-22 Gauge 1-1 1/2 in Needle (Draw) 1 - 22 ...
-
SPL UNCLASSIFIED SECTIONAssembled and Distributed by IT3 Medical, LLC - 190 E Stacy Road; STE 306-298 Allen, TX 75002-8734 - For questions or comments: info@IT3-Medical.com, www.IT3-Medical.com
-
Packaging

-
INGREDIENTS AND APPEARANCEProduct Information