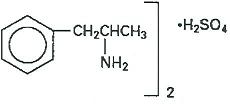
Information for Patients
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
-
Educate patients and their families about the risks of ...
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of dextroamphetamine sulfate which can lead to overdose and death, and proper disposal of any unused drug (see WARNINGS, DRUG ABUSE AND DEPENDENCE, and OVERDOSAGE). Advise patients to store dextroamphetamine sulfate in a safe place, preferably locked, and instruct patients to not give dextroamphetamine sulfate to anyone else.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with dextroamphetamine sulfate use. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease (see WARNINGS).
Increased Blood Pressure and Heart Rate
Advise patients that dextroamphetamine sulfate can elevate blood pressure and heart rate (see WARNINGS).
Psychiatric Adverse Reactions
Advise patients that dextroamphetamine sulfate, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania (see WARNINGS).
Long-Term Suppression of Growth in Pediatric Patients
Advise patients that dextroamphetamine sulfate, may cause slowing of growth including weight loss (see WARNINGS).
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]
- Instruct patients beginning treatment with dextroamphetamine sulfate oral solution about the risk of peripheral vasculopathy, including Raynaud’s Phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking dextroamphetamine oral solution.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with concomitant use of dextroamphetamine sulfate and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others such as linezolid [see CONTRAINDICATIONS, WARNINGS, and DRUG INTERACTIONS]. Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with dextroamphetamine sulfate. Instruct the patients to notify their healthcare provider if emergence or worsening of tics or Tourette’s syndrome occurs (see WARNINGS).
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or vehicles; the patient should therefore be cautioned accordingly.
Drug Interactions
MAO Inhibitors - MAOI antidepressants, as well as a metabolite of furazolidone, slow amphetamine metabolism. This slowing potentiates amphetamines, increasing their effect on the release of norepinephrine and other monoamines from adrenergic nerve endings; this can cause headaches and other signs of hypertensive crisis. A variety of neurological toxic effects and malignant hyperpyrexia can occur, sometimes with fatal results.
Serotonergic Drugs - The concomitant use of dextroamphetamine sulfate oral solution and serotonergic drugs increases the risk of serotonin syndrome. Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during dextroamphetamine sulfate oral solution initiation or dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine sulfate oral solution and the concomitant serotonergic drug(s) [see WARNINGS, PRECAUTIONS]. Examples of serotonergic drugs include selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John’s Wort.
CYP2D6 Inhibitors - The concomitant use of dextroamphetamine sulfate oral solution and CYP2D6 inhibitors may increase the exposure of dextroamphetamine sulfate oral solution compared to the use of the drug alone and increase the risk of serotonin syndrome. Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome particularly during dextroamphetamine sulfate oral solution initiation and after a dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine sulfate oral solution and the CYP2D6 inhibitor [see WARNINGS, OVERDOSAGE]. Examples of CYP2D6 Inhibitors include paroxetine and fluoxetine (also serotonergic drugs), quinidine, ritonavir.
Acidifying Agents - Gastrointestinal acidifying reagents (guanethidine, reserpine, glutamic acid HCl, ascorbic acid, fruit juices, etc.) lower absorption of amphetamines. Urinary acidifying agents (ammonium chloride, sodium acid phosphate, etc.) increase the concentration of the ionized species of the amphetamine molecule, thereby increasing urinary excretion. Both groups of agents lower blood levels and efficacy of amphetamines.
Adrenergic Blockers - Adrenergic blockers are inhibited by amphetamines.
Alkalinizing Agents - Gastrointestinal alkalinizing agents (sodium bicarbonate, etc.) increase absorption of amphetamines. Urinary alkalinizing agents (acetazolamide, some thiazides) increase the concentration of the non-ionized species of the amphetamine molecule, thereby decreasing urinary excretion. Both groups of agents increase blood levels and therefore potentiate the actions of amphetamines.
Antidepressants, Tricyclic - Amphetamines may enhance the activity of tricyclic or sympathomimetic agents; d-amphetamine with desipramine or protriptyline and possibly other tricyclics cause striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated.
Antihistamines - Amphetamines may counteract the sedative effect of antihistamines.
Antihypertensives - Amphetamines may antagonize the hypotensive effects of antihypertensives.
Chlorpromazine - Chlorpromazine blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines, and can be used to treat amphetamine poisoning.
Ethosuximide - Amphetamines may delay intestinal absorption of ethosuximide.
Haloperidol - Haloperidol blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines.
Lithium Carbonate - The stimulatory effects of amphetamines may be inhibited by lithium carbonate.
Meperidine - Amphetamines potentiate the analgesic effect of meperidine.
Methenamine Therapy - Urinary excretion of amphetamines is increased, and efficacy is reduced, by acidifying agents used in methenamine therapy.
Norepinephrine - Amphetamines enhance the adrenergic effect of norepinephrine.
Phenobarbital - Amphetamines may delay intestinal absorptions of phenobarbital; co-administration of phenobarbital may produce a synergistic anticonvulsant action.
Phenytoin - Amphetamines may delay intestinal absorption of phenytoin; co-administration of phenytoin may produce a synergistic anticonvulsant action.
Propoxyphene - In cases of propoxyphene overdosage, amphetamine CNS stimulation is potentiated and fatal convulsions can occur.
Veratrum Alkaloids - Amphetamines inhibit the hypotensive effect of veratrum alkaloids.
Drug/Laboratory Test Interactions
Amphetamines can cause a significant elevation in plasma corticosteroid levels. This increase is greatest in the evening.
Amphetamines may interfere with urinary steroid determinations.
Carcinogenesis/Mutagenesis
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of dextroamphetamine sulfate have not been performed.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Dextroamphetamine sulfate has been shown to have embryotoxic and teratogenic effects when administered to A/Jax mice and C57BL mice in doses approximately 41 times the maximum human dose. Embryotoxic effects were not seen in New Zealand white rabbits given the drug in doses 7 times the human dose nor in rats given 12.5 times the maximum human dose. While there are no adequate and well-controlled studies in pregnant women, there has been one report of severe congenital bony deformity, tracheoesophageal fistula, and anal atresia (VATER association) in a baby born to a woman who took dextroamphetamine sulfate with lovastatin during the first trimester of pregnancy. Dextroamphetamine sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation, and significant lassitude.
Nursing Mothers
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use
Long-term effects of amphetamines in pediatric patients have not been well established.
Amphetamines are not recommended for use in pediatric patients under 3 years of age with Attention Deficit Disorder with Hyperactivity described under
INDICATIONS AND USAGE.
Clinical experience suggests that in psychotic children, administration of amphetamines may exacerbate symptoms of behavior disturbance and thought disorder.
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette’s syndrome. Therefore, clinical evaluation for tics and Tourette’s syndrome in children and their families should precede use of stimulant medications.
Data are inadequate to determine whether chronic administration of amphetamines may be associated with growth inhibition; therefore, growth should be monitored during treatment.
Drug treatment is not indicated in all cases of Attention Deficit Disorder with Hyperactivity and should be considered only in light of the complete history and evaluation of the child. The decision to prescribe amphetamines should depend on the physician’s assessment of the chronicity and severity of the child’s symptoms and their appropriateness for his or her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with amphetamines is usually not indicated.
Close