Label: PRETOMANID tablet
-
NDC Code(s):
49502-476-14,
49502-476-26,
49502-476-29,
49502-476-32, view more49502-476-33, 49502-476-72
- Packager: Viatris Specialty LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRETOMANID TABLETS safely and effectively. See full prescribing information for PRETOMANID TABLETS. PRETOMANID tablets, for oral ...These highlights do not include all the information needed to use PRETOMANID TABLETS safely and effectively. See full prescribing information for PRETOMANID TABLETS.
PRETOMANID tablets, for oral use
Initial U.S. Approval: 2019
LIMITED POPULATIONRECENT MAJOR CHANGES
INDICATIONS AND USAGE
Limited Population: Pretomanid Tablet is an antimycobacterial indicated, as part of a combination regimen with bedaquiline and linezolid for the treatment of adults with pulmonary tuberculosis (TB) that is resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug OR adults with pulmonary TB resistant to isoniazid and rifampin, who are treatment-intolerant or nonresponsive to standard therapy. Approval of this indication is based on limited clinical safety and efficacy data. This drug is indicated for use in a limited and specific population of patients. (1)
Limitations of Use: (1)
Pretomanid Tablets are not indicated in patients with:
- •
- Drug-sensitive (DS) TB
- •
- Latent infection due to Mycobacterium tuberculosis
- •
- Extra-pulmonary infection due to Mycobacterium tuberculosis
- •
- TB resistant to isoniazid and rifampin, who are responsive to standard therapy and not treatment-intolerant
- •
- TB with known resistance to any component of the combination
Safety and effectiveness of Pretomanid Tablets have not been established for its use in combination with drugs other than bedaquiline and linezolid as part of the recommended dosing regimen.
DOSAGE AND ADMINISTRATION
Important Administration Instructions: (2.1)
- •
- Pretomanid Tablets must be administered only in combination with bedaquiline and linezolid as part of the recommended dosage regimen.
- •
- Administer the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid by directly observed therapy (DOT).
- •
- Pretomanid Tablets must be administered in combination with bedaquiline and linezolid with food.
- •
- Doses of the combination regimen missed for safety reasons can be made up at the end of treatment; doses of linezolid alone missed due to linezolid adverse reactions should not be made up.
- •
- Administer Pretomanid Tablets in combination with bedaquiline and linezolid as follows: (2.2)
- •
-
Pretomanid Tablets:
- •
- Pretomanid Tablet 200 mg orally once daily for 26 weeks. Administer Pretomanid Tablets whole with water.
- •
- For patients with swallowing difficulties see the full prescribing information for crushing or soaking followed by crushing instructions of the Pretomanid Tablets.
- •
-
Bedaquiline:
- •
- Bedaquiline 400 mg orally once daily for 2 weeks followed by 200 mg 3 times per week, with at least 48 hours between doses, for 24 weeks for a total of 26 weeks.
- •
- Bedaquiline 200 mg orally once daily for 8 weeks followed by 100 mg once daily for 18 weeks, for a total of 26 weeks.
- •
-
Linezolid:
- •
- Linezolid Preferred: 600 mg orally once daily for 26 weeks. Alternative: 1,200 mg orally once daily for 26 weeks.
- •
- If myelosuppression, peripheral neuropathy, or optic neuropathy occurs, reduce or interrupt linezolid dosing as necessary.
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg (3)
CONTRAINDICATIONS
- •
- Pretomanid Tablets used in combination with bedaquiline and linezolid are contraindicated in patients for whom bedaquiline and/or linezolid is contraindicated. (4)
WARNINGS AND PRECAUTIONS
- •
- Hepatic adverse reactions were reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor symptoms and signs and liver‑related laboratory tests. Interrupt treatment with the entire regimen if evidence of liver injury occurs. (5.2)
- •
- Myelosuppression was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor complete blood counts. Decrease or interrupt linezolid dosing if significant myelosuppression develops or worsens. (5.3)
- •
- Peripheral and optic neuropathy were reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor visual function. Obtain an ophthalmologic evaluation if there are symptoms of visual impairment. Decrease or interrupt linezolid dosing if neuropathy develops or worsens. (5.4)
- •
- QT prolongation was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Use with drugs that prolong the QT interval may cause additive QT prolongation. Monitor ECGs. Discontinue the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if significant ventricular arrhythmia or if QTcF interval prolongation of greater than 500 ms develops. (5.5)
- •
- Reproductive effects: Pretomanid caused testicular atrophy and impaired fertility in male rats. Advise patients of reproductive toxicities seen in animal studies and that the potential effects on human male fertility have not been adequately evaluated. (5.7, 13.1)
- •
- Lactic acidosis was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Consider interrupting linezolid or the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid dosing if significant lactic acidosis develops. (5.8)
ADVERSE REACTIONS
Most common adverse reactions (≥ 10%) are peripheral neuropathy, anemia, nausea, acne, vomiting, increased transaminases, headache, musculoskeletal pain, dyspepsia, rash, pruritus, decreased appetite, abdominal pain, pleuritic pain, increased gamma-glutamyltransferase, hemoptysis and hyperamylasemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Assessments Prior to Initiating the Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid
2.4 Discontinuation of Dosing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with the Combination Treatment Regimen
5.2 Hepatotoxicity
5.3 Myelosuppression
5.4 Peripheral and Optic Neuropathy
5.5 QT Prolongation
5.6 Drug Interactions
5.7 Reproductive Effects
5.8 Lactic Acidosis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Pretomanid
7.2 Effect of Pretomanid on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE Limited Population: Pretomanid Tablet is indicated, as part of a combination regimen with bedaquiline and linezolid for the treatment of adults with pulmonary tuberculosis (TB) resistant to ...
Limited Population: Pretomanid Tablet is indicated, as part of a combination regimen with bedaquiline and linezolid for the treatment of adults with pulmonary tuberculosis (TB) resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug OR adults with pulmonary TB resistant to isoniazid and rifampin, who are treatment-intolerant or nonresponsive to standard therapy. Approval of this indication is based on limited clinical safety and efficacy data. This drug is indicated for use in a limited and specific population of patients.
Limitations of Use:
- •
- Pretomanid Tablets are not indicated in patients with:
- o
- Drug-sensitive (DS) TB
- o
- Latent infection due to Mycobacterium tuberculosis
- o
- Extra-pulmonary infection due to Mycobacterium tuberculosis
- o
- TB resistant to isoniazid and rifampin who are responsive to standard therapy and not treatment-intolerant
- o
- TB with known resistance to any component of the combination
- •
- Safety and effectiveness of Pretomanid Tablets have not been established for its use in combination with drugs other than bedaquiline and linezolid as part of the recommended dosing regimen [see Dosage and Administration (2.2)].
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions - • Pretomanid Tablets must be administered only in combination with bedaquiline and linezolid as part of the recommended dosage regimen [see Dosage ...
2.1 Important Administration Instructions
- •
- Pretomanid Tablets must be administered only in combination with bedaquiline and linezolid as part of the recommended dosage regimen [see Dosage and Administration (2.2)].
- •
- Administer the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid by directly observed therapy (DOT).
- •
- Pretomanid Tablets must be administered in combination with bedaquiline and linezolid with food [see Clinical Pharmacology (12.3)].
- •
- Emphasize the need for compliance with the full course of therapy to patients [see Patient Counseling Information (17)].
- •
- If the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid is interrupted by a healthcare provider for safety reasons, missed doses can be made up at the end of the treatment; doses of linezolid alone missed due to linezolid adverse reactions should not be made up [see Dosage and Administration (2.4)].
- •
- Dosing of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can be extended beyond 26 weeks, if necessary [see Clinical Studies (14)].
2.2 Recommended Dosage
Pretomanid Tablets must be administered in combination with bedaquiline and linezolid with food [see Clinical Pharmacology (12.3)]. The recommended dosage and duration for Pretomanid Tablets, bedaquiline and linezolid when administered in the combination regimen are as follows:
Pretomanid Tablets:
- •
- Administer Pretomanid Tablet 200 mg orally (1 tablet of 200 mg), once daily, for 26 weeks with food.
- •
- Administer Pretomanid Tablets whole with water.
- •
- For adult patients with swallowing difficulties, use one of the following two methods:
- A)
- Crush and Suspend Pretomanid Tablets
- •
- Crush and suspend Pretomanid Tablet in one teaspoon (approximately 5 mL) of room temperature water in a drinking cup and mix well by vigorous stirring.
- •
- Orally administer the contents of the cup immediately.
- •
- To ensure no tablet residual is left in the cup, rinse with an additional one teaspoon (approximately 5 mL) of water and orally administer the contents of the cup immediately.
- •
- Do not store the mixture for later use.
- B)
- Soak and then Crush Pretomanid Tablets
- •
- Soak Pretomanid Tablet for 4 to 5 minutes in one teaspoon (approximately 5 mL) of room temperature water in a drinking cup and then any remaining solid should be crushed. Mix the contents of the cup well by vigorous stirring.
- •
- Orally administer the contents of the cup immediately.
- •
- To ensure no tablet residual is left in the cup, rinse with an additional one teaspoon (approximately 5 mL) of water and orally administer the contents of the cup immediately.
- •
- Do not store the mixture for later use.
Bedaquiline:
Use one of the following two dosage regimens of bedaquiline:
- •
- Bedaquiline 400 mg orally once daily for 2 weeks followed by 200 mg 3 times per week, with at least 48 hours between doses, for 24 weeks, for a total of 26 weeks.
- •
- Bedaquiline 200 mg orally once daily for 8 weeks followed by 100 mg once daily for 18 weeks, for a total of 26 weeks.
Linezolid:
- •
- Preferred linezolid dosage regimen: 600 mg orally once daily for 26 weeks. If myelosuppression, peripheral neuropathy, or optic neuropathy occurs, reduce linezolid dosage to 300 mg once daily or interrupt linezolid dosing [see Dosage and Administration (2.4), Warnings and Precautions (5.3, 5.4), and Adverse Reactions (6.1)].
- •
- Alternative linezolid dosage regimen: 1,200 mg orally once daily for 26 weeks. If myelosuppression, peripheral neuropathy, or optic neuropathy occurs, reduce linezolid dosage to 600 mg once daily, further reduce linezolid dosage to 300 mg once daily, or interrupt linezolid dosing [see Dosage and Administration (2.4), Warnings and Precautions (5.3, 5.4), and Adverse Reactions (6.1)].
2.3 Assessments Prior to Initiating the Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid
- •
- Assess for symptoms and signs of liver disease (such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, and hepatomegaly). Obtain laboratory tests (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and bilirubin) [see Warnings and Precautions (5.2)].
- •
- Obtain complete blood count [see Warnings and Precautions (5.3)]. Obtain serum potassium, calcium, and magnesium and correct if abnormal [see Warnings and Precautions (5.5)]. Obtain an ECG before initiation of treatment [see Warnings and Precautions (5.5)].
Close2.4 Discontinuation of Dosing
If either bedaquiline or Pretomanid Tablets are discontinued, the entire combination regimen should also be discontinued.
If linezolid is permanently discontinued during the initial four consecutive weeks of treatment, bedaquiline and Pretomanid Tablets should also be discontinued. If linezolid is discontinued after the initial four weeks of consecutive treatment, continue administering bedaquiline and Pretomanid Tablets [see Dosage and Administration (2.2)].
-
3 DOSAGE FORMS AND STRENGTHS Pretomanid Tablets, 200 mg, are white to off-white, oval tablets debossed with M on one side and P200 on the other side.
Pretomanid Tablets, 200 mg, are white to off-white, oval tablets debossed with M on one side and P200 on the other side.
Close -
4 CONTRAINDICATIONS Pretomanid Tablets used in the combination regimen with bedaquiline and linezolid are contraindicated in patients for whom bedaquiline and/or linezolid are contraindicated. Refer to the ...
Pretomanid Tablets used in the combination regimen with bedaquiline and linezolid are contraindicated in patients for whom bedaquiline and/or linezolid are contraindicated. Refer to the bedaquiline and linezolid prescribing information.
Close -
5 WARNINGS AND PRECAUTIONS 5.1 Risks Associated with the Combination Treatment Regimen - Pretomanid Tablet is indicated for use as part of a regimen in combination with bedaquiline and linezolid. Refer to the prescribing ...
5.1 Risks Associated with the Combination Treatment Regimen
Pretomanid Tablet is indicated for use as part of a regimen in combination with bedaquiline and linezolid. Refer to the prescribing information for bedaquiline and linezolid for additional risk information. Warnings and Precautions related to bedaquiline and linezolid also apply to their use in the combination regimen with Pretomanid Tablets.
5.2 Hepatotoxicity
Hepatic adverse reactions were reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. Avoid alcohol and hepatotoxic agents, including herbal supplements and drugs other than bedaquiline and linezolid [see Indications and Usage (1)] while on Pretomanid Tablets, especially in patients with impaired hepatic function.
Monitor symptoms and signs (such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, and hepatomegaly) and laboratory tests (ALT, AST, alkaline phosphatase, and bilirubin) at a minimum at baseline, at two weeks, and then monthly while on treatment and as needed. If evidence of new or worsening liver dysfunction occurs, test for viral hepatitides and discontinue other hepatotoxic medications. Interrupt treatment with the entire regimen if:
- •
- Aminotransferase elevations are accompanied by total bilirubin elevation greater than 2 times the upper limit of normal.
- •
- Aminotransferase elevations are greater than 8 times the upper limit of normal.
- •
- Aminotransferase elevations are greater than 5 times the upper limit of normal and persist beyond 2 weeks.
5.3 Myelosuppression
Myelosuppression (including anemia, leukopenia, thrombocytopenia, and pancytopenia) was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Myelosuppression is a known adverse reaction of linezolid. Anemia can be life threatening [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. When linezolid dosing, as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, was reduced, interrupted, or discontinued, the observed hematologic abnormalities were reversible. Complete blood counts should be monitored at a minimum at baseline, at two weeks, and then monthly in patients receiving linezolid as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Decreasing linezolid to half the initial dose or interrupting linezolid dosing should be considered in patients who develop or have worsening myelosuppression [see Dosage and Administration (2.2)].
5.4 Peripheral and Optic Neuropathy
Peripheral neuropathy and optic neuropathy were reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)]. Neuropathy is a known adverse reaction of long-term linezolid use. Neuropathy associated with linezolid is generally reversible or improved with appropriate monitoring and interruption, dose reduction, or discontinuation of linezolid dosing. When improvement in the peripheral neuropathy is observed, consider resuming linezolid at half the initial dose [see Dosage and Administration (2.2)]. Monitor visual function in all patients receiving the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid; if a patient experiences symptoms of visual impairment, interrupt linezolid dosing and obtain prompt ophthalmologic evaluation.
5.5 QT Prolongation
QT prolongation was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Pharmacology (12.2)]. QT prolongation is a known adverse reaction of bedaquiline. Obtain an ECG before initiation of treatment, and at least 2, 12, and 24 weeks after starting treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Obtain serum potassium, calcium, and magnesium at baseline and correct if abnormal. Monitor these electrolytes if QT prolongation is detected [see Adverse Reactions (6.1)].
The following may increase the risk for QT prolongation when patients are receiving bedaquiline as part of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid: a history of Torsade de Pointes, congenital long QT syndrome, ongoing hypothyroidism, ongoing bradyarrhythmia, uncompensated heart failure, or serum calcium, magnesium, or potassium levels below the lower limits of normal. If necessary, bedaquiline treatment initiation could be considered in these patients after a favorable benefit-risk assessment and with frequent ECG monitoring.
Discontinue the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if the patient develops clinically significant ventricular arrhythmia or a QTcF interval of greater than 500 ms (confirmed by repeat ECG). If syncope occurs, obtain an ECG to detect QT prolongation.
5.6 Drug Interactions
CYP3A4 Inducers
Pretomanid may be in part metabolized by CYP3A4 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Avoid co‑administration of strong or moderate CYP3A4 inducers, such as rifampin or efavirenz, during treatment with pretomanid.
5.7 Reproductive Effects
Pretomanid caused testicular atrophy and impaired fertility in male rats. Advise patients of reproductive toxicities seen in animal studies and that the potential effects on human male fertility have not been adequately evaluated [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
Close5.8 Lactic Acidosis
Lactic acidosis was reported with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Lactic acidosis is a known adverse reaction of linezolid. Patients who develop recurrent nausea or vomiting should receive immediate medical evaluation, including evaluation of bicarbonate and lactic acid levels, and interruption of linezolid or the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid should be considered.
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed here and elsewhere in the labeling: • Hepatotoxicity [see Warnings and Precautions (5.2)] • Myelosuppression [see Warnings and Precautions ...
The following serious adverse reactions are discussed here and elsewhere in the labeling:
- •
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- •
- Myelosuppression [see Warnings and Precautions (5.3)]
- •
- Peripheral and Optic Neuropathy [see Warnings and Precautions (5.4)]
- •
- QT Prolongation [see Warnings and Precautions (5.5)]
- •
- Reproductive Effects [see Warnings and Precautions (5.7)]
- •
- Lactic Acidosis [see Warnings and Precautions (5.8)]
Close6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, refer to the prescribing information for the respective drugs for a description of the adverse reactions associated with their use.
Approximately 3100 subjects have been exposed to Pretomanid Tablets, either alone or as part of a combination therapy in clinical trials.
The registrational trial, Trial 1 (NCT02333799), was a single-arm, open-label trial conducted in three sites in South Africa in which adult patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug or pulmonary TB resistant to isoniazid and rifampin, who were treatment-intolerant or non-responsive to standard therapy received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 6 months (extendable to 9 months) with 24 months of follow-up. One hundred and nine patients were treated; 76% were black, and 23% were of mixed race. Their ages ranged from 17 years to 60 years (mean 36 years), and all patients were from South Africa. Fifty-six (51%) patients were HIV-positive. There were 8 deaths. Six patients died while receiving treatment; all surviving patients, excluding one patient who withdrew consent, completed treatment. Two patients died during follow-up at Day 369 and Day 486, respectively.
Trial 2 (NCT03086486) was a phase 3 partially-blinded, randomized trial assessing the safety and efficacy of various doses and treatment durations of linezolid in combination with Pretomanid Tablets plus bedaquiline in patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug, or pulmonary TB resistant to rifamycins and either a fluoroquinolone or a second line injectable antibacterial drug, or pulmonary TB resistant to isoniazid and rifampin who were treatment intolerant or non-responsive to standard therapy.
A total of 181 patients were randomized to one of the 4 treatment arms, Pretomanid Tablets plus bedaquiline plus either 1,200 mg or 600 mg of linezolid for 26 weeks or for 9 weeks (not an approved dosing regimen), followed by a linezolid placebo for 17 weeks. Males represented 67% of the patients in the trial and the median age of all patients was 36 years. 64% of patients were White and the remaining patients were Black (36%). The treatment groups were comparable with respect to demographic characteristics. Most patients (93%) completed treatment. One patient died in the linezolid 1,200 mg for 9 weeks (not an approved dosing regimen) arm.
Common Adverse Reactions Reported in Trials 1 and 2
Table 1 summarizes the incidence of select adverse reactions occurring in ≥ 5% of patients treated for 26 weeks in Trials 1 and 2.
Table 1: Select Adverse Reactions (All Grades) Reported in ≥ 5% of Patients Receiving the Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid in Trials 1 and 2 - *
- Select terms are collapsed, as follows: peripheral neuropathy (burning sensation, hypoesthesia, hyporeflexia, neuropathy peripheral, paresthesia, peripheral motor neuropathy, peripheral sensorimotor neuropathy, peripheral sensory neuropathy, polyneuropathy, sensory disturbance); anemia (anemia); acne (acne, dermatitis acneiform); transaminases increased (alanine aminotransferase [ALT]) increased, aspartate aminotransferase [AST] increased, drug-induced liver injury, hepatic enzyme increased, hepatic function abnormal, liver function test increased, transaminases increased); musculoskeletal pain (arthralgia, back pain, costochondritis, myalgia, pain in extremity); rash (rash, rash erythematous, rash maculo-papular, rash papular, rash vesicular); pruritus (pruritus, pruritus generalized, rash pruritic); abdominal pain (abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness); hyperamylasemia (amylase increased, hyperamylasemia); hypertension (blood pressure increased, hypertension); cough (cough, productive cough); visual impairment (vision blurred, visual acuity reduced, visual impairment); neutropenia (neutropenia); hyperlipasemia (hyperlipasemia, lipase increased).
Pretomanid Tablets, Bedaquiline and Linezolid (1,200 mg)
Combination Regimen
(N = 154)Pretomanid Tablets, Bedaquiline and Linezolid (600 mg)
Combination Regimen
(N = 45)Adverse Reactions
All Grades
n (%)All Grades
n (%)Peripheral neuropathy*
105 (68)
10 (22)
Anemia*
48 (31)
Nausea
46 (30)
Acne*
44 (29)
6 (13)
Vomiting
40 (26)
Transaminases increased*
39 (25)
11 (24)
Headache
34 (22)
Musculoskeletal pain*
34 (22)
Dyspepsia
30 (19)
Rash*
29 (19)
Pruritus*
25 (16)
3 (7)
Decreased appetite
24 (16)
Abdominal pain*
22 (14)
Pleuritic pain
22 (14)
4 (9)
Gamma-glutamyltransferase increased
20 (13)
Hemoptysis
17 (11)
Hyperamylasemia*
15 (10)
Diarrhea
14 (9)
Hypertension*
14 (9)
Cough*
13 (8)
Visual impairment*
13 (8)
Hypoglycemia
12 (8)
Neutropenia*
12 (8)
3 (7)
Abnormal loss of weight
11 (7)
Constipation
9 (6)
Gastritis
9 (6)
Hyperlipasemia*
9 (6)
Insomnia
9 (6)
Dry skin
8 (5)
The following select adverse reactions were reported in patients receiving the combination regimen of Pretomanid Tablets, bedaquiline and linezolid (1,200 mg) at a rate of less than 5% in Trials 1 and 2:
Gastrointestinal Disorders: pancreatitis, dysgeusia
Investigations: blood creatine phosphokinase increase, blood creatinine increase, blood alkaline phosphatase increase
Blood and Lymphatic System Disorders: leukopenia, thrombocytopenia
Metabolism and Nutrition Disorders: hypomagnesemia, hyperglycemia, hypokalemia, hyperkalemia, hyponatremia
Nervous System Disorders: dizziness, seizure
The following select adverse reactions were reported in patients receiving the combination regimen of Pretomanid Tablets, bedaquiline and linezolid (600 mg) at a rate of less than 5% in Trial 2:
Blood and Lymphatic System Disorders: anemia, thrombocytopenia
Metabolism and Nutrition Disorders: decreased appetite, hyperlipasemia, hypoglycemia, hypophosphatemia, hypomagnesemia, hyperkalemia
Psychiatric Disorders: insomnia
Nervous System Disorders: headache
Vascular Disorders: hypertension
Respiratory, Thoracic and Mediastinal Disorders: cough, hemoptysis
Gastrointestinal Disorders: abdominal pain, constipation, diarrhea, dyspepsia, nausea, vomiting
Skin and Subcutaneous Tissue Disorders: dry skin, rash
Musculoskeletal and Connective Tissue Disorders: musculoskeletal pain
Laboratory Abnormalities Reported in Trials 1 and 2
Table 2 summarizes select laboratory abnormalities in patients treated for 26 weeks in Trials 1 and 2.
Table 2: Select Laboratory Abnormalities in Clinical Trials ULN = upper limit of normal Parameter
Multiples of Upper Limit of Normal (x ULN)
Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid (1,200 mg)
(N = 154)
n (%)Combination Regimen of Pretomanid Tablets, Bedaquiline, and Linezolid (600 mg)
(N = 45)
n (%)Transaminases and Bilirubin
Alanine Aminotransferase (ALT)
> 3 and ≤ 5 X ULN
13 (8)
3 (7)
> 5 and ≤ 8 X ULN
6 (4)
0 (0)
> 8 X ULN
1 (1)
1 (2)
Aspartate Aminotransferase (AST)
> 3 and ≤ 5 X ULN
7 (5)
0 (0)
> 5 and ≤ 8 X ULN
3 (2)
0 (0)
> 8 X ULN
1 (1)
1 (2)
Total Bilirubin
> 1 X ULN and ≤ 2 X ULN
8 (5)
1 (2)
> 2 X ULN
2 (1)
1 (2)
Hematology
Hemoglobin
≤ 7.9 g/dL
6 (4)
0 (0)
Neutrophils Absolute Count
≤ 749/mm3
6 (4)
1 (2)
Platelets
≤ 49,999/mm3
2 (1)
0 (0)
Serum Chemistry
Lipase
> 2 X ULN
7 (5)
2 (4)
In Trial 1, 28% of patients experienced increased transaminases. Except for one patient who died due to pneumonia and sepsis, all patients who experienced increased transaminases were able to continue therapy and complete the full course of treatment. In Trial 2, 47 of 181 patients (26%) had one or more liver-related adverse reactions, with similar numbers in each group.
Myelosuppression is a known adverse reaction of linezolid. In Trial 1, the most common hematopoietic cytopenia was anemia (37%). The majority of cytopenias began after 2 weeks of treatment. Three patients experienced cytopenias that were considered serious: neutropenia in 1 patient and anemia in 2 patients. All 3 serious adverse reactions resulted in interruption of linezolid or all components of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, and all resolved.
In Trial 2, there was a greater incidence of myelosuppression, 29% versus 13%, for the 1,200 mg compared to the 600 mg linezolid 26-week group. Most of the myelosuppression-related adverse reactions were either grade 1 or grade 2 in severity.
In the combined study data for Trial 1 and Trial 2, 2 patients reported serious adverse reactions of anemia with linezolid 1,200 mg, and none were reported in the 600 mg group.
Peripheral and Optic Neuropathy
Peripheral neuropathy is a known adverse reaction of linezolid. In Trial 1, peripheral neuropathy was reported in 81% of patients. Most of these adverse reactions (64%) occurred after 8 weeks of treatment and resulted in dosing interruption, dose reduction, or discontinuation of linezolid. Severe, moderate, and mild peripheral neuropathy occurred in 22%, 32%, and 26% of patients, respectively. No adverse reaction related to peripheral neuropathy led to a discontinuation of the entire study regimen.
In Trial 2, 17 (38%) patients reported an adverse reaction of peripheral neuropathy in the 1,200 mg 26-week treatment group; one of these reactions led to treatment discontinuation. In the 600 mg 26-week treatment group, 10 (22%) patients reported peripheral neuropathy, and none required linezolid treatment interruption or treatment discontinuation.
Optic neuropathy is a known adverse reaction of linezolid. Two patients (2%) in Trial 1 developed optic neuropathy after 16 weeks of treatment. Both were serious, confirmed on retinal examination as optic neuropathy/neuritis, and resulted in discontinuation of linezolid; both adverse reactions resolved.
Overall, patients administered a linezolid dose of 600 mg twice daily had a similar safety profile to those administered a dose of 1,200 mg once daily.
In Trial 2 overall, 4 (2%) patients reported an adverse reaction of optic neuropathy. All 4 patients were in the 1,200 mg linezolid 26-week treatment group (9%). The maximum severity was grade 1 (mild) for 1 patient, grade 2 (moderate) for 2 patients, and grade 3 (severe) for 1 patient. All patients had linezolid permanently discontinued except 1 patient who had already completed treatment when the adverse reaction occurred. Onset of optic neuropathy occurred after 3 months of treatment, and resolved.
-
7 DRUG INTERACTIONS 7.1 Effect of Other Drugs on Pretomanid CYP3A4 Inducers - Co-administration of pretomanid with rifampin and efavirenz resulted in a decrease in pretomanid plasma concentrations [see ...
7.1 Effect of Other Drugs on Pretomanid
CYP3A4 Inducers
Co-administration of pretomanid with rifampin and efavirenz resulted in a decrease in pretomanid plasma concentrations [see Clinical Pharmacology (12.3)]. Avoid co-administration of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid with rifampin, efavirenz, or other strong or moderate CYP3A4 inducers. Refer to the prescribing information for bedaquiline for additional information about drug interactions with CYP3A4.
Lopinavir/Ritonavir
Co-administration of pretomanid with lopinavir/ritonavir did not affect the plasma concentrations of pretomanid [see Clinical Pharmacology (12.3)]. Lopinavir/ritonavir can be co-administered with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
Close7.2 Effect of Pretomanid on Other Drugs
Midazolam
Co-administration of pretomanid with the CYP3A4 substrate, midazolam, resulted in no clinically significant effect on the pharmacokinetics of midazolam or its major metabolite, 1-hydroxy-midazolam [see Clinical Pharmacology (12.3)]. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can be administered with CYP3A4 substrate drugs.
Organic Anion Transporter-3 (OAT3), BCRP, OATP1B3 and P-gp Substrates
The effect of co-administration of pretomanid on the pharmacokinetics of OAT3 substrates in humans is unknown. However, in vitro studies indicate that pretomanid significantly inhibits the OAT3 drug transporter [see Clinical Pharmacology (12.3)], which could result in increased concentrations of OAT3 substrate drugs clinically and may increase the risk of adverse reactions associated with these drugs.
If pretomanid is co-administered with OAT3 substrate drugs (e.g., methotrexate, indomethacin, ciprofloxacin), increase monitoring for OAT3 substrate drug-related adverse reactions and consider dosage reduction for OAT3 substrate drugs, if needed. Refer to the prescribing information of the co-administered drug for dosage reduction information.
In vitro studies cannot exclude the possibility that pretomanid is an inhibitor of BCRP, OATP1B3 and P-gp [see Clinical Pharmacology (12.3)]. No clinical studies have been performed to investigate these interactions. Therefore, it cannot be excluded that co-administration of pretomanid with sensitive OATP1B3 substrates (e.g., valsartan, statins), BCRP substrates (e.g., rosuvastatin, prazosin, glyburide, sulfasalazine) and P-gp substrates (e.g., digoxin, dabigatran etexilate, verapamil) may increase their exposure. If pretomanid is co-administered with substrates of OATP1B3, BCRP, or P-gp, increased monitoring for drug-related adverse reactions to the co-administered medicinal product should be performed.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no studies or available data on pretomanid use in pregnant women to inform any drug-associated risks. There are risks associated with active ...
8.1 Pregnancy
Risk Summary
There are no studies or available data on pretomanid use in pregnant women to inform any drug-associated risks. There are risks associated with active tuberculosis during pregnancy (see Clinical Considerations). When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, the pregnancy information for bedaquiline and linezolid also applies to this combination regimen. Refer to the bedaquiline and linezolid prescribing information for more information on bedaquiline and linezolid associated risks of use during pregnancy. In animal reproduction studies, there was increased post-implantation loss in the presence of maternal toxicity (reduced bodyweight and feed consumption) with oral administration of pretomanid during organogenesis in rats at doses about 4 times the exposure at the recommended dose in humans. There were no adverse embryo fetal effects in rats or rabbits dosed with oral pretomanid during organogenesis at doses up to approximately 2 times the exposure in humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Animal Data
In animal reproduction studies, pregnant rats were dosed orally with pretomanid at 10, 30, and 100 mg/kg/day during organogenesis (gestational Days 7 through 17). Rats showed increased post-implantation loss in the presence of maternal toxicity (including reduced body weight and feed consumption) at 100 mg/kg/day, approximately 4 times the exposure in humans for a 200 mg dose on an AUC basis. There were no adverse embryofetal effects in rats dosed with oral pretomanid during organogenesis at doses up to approximately 2 times the exposure in humans. Pregnant rabbits were dosed orally with pretomanid during organogenesis (gestational Days 7 through 19) at 10, 30, and 60 mg/kg/day. No evidence of adverse developmental outcomes was observed when oral doses of pretomanid were administered to dams during organogenesis (gestational Days 7 to 19) at doses up to 60 mg/kg/day (approximately 2 times the exposure in humans for a 200 mg dose on an AUC basis).
In a pre- and postnatal development study, there were no adverse developmental effects in pups of pregnant rats orally dosed with up to 20 mg/kg/day from gestational Day 6 through lactation Day 20. Pups of pregnant females dosed at 60 mg/kg/day (about 2 times the exposure for the 200 mg dose) had lower body weights and a slight delay in the age at which the air-drop righting reflex developed. These effects occurred at a maternally toxic dose (based on maternal weight loss and reduced food consumption).
8.2 Lactation
Risk Summary
There is no information regarding the presence of pretomanid in human milk, or its effects on milk production or the breastfed infant. Pretomanid was detected in rat milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for adverse reactions in nursing infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Pretomanid Tablets and any potential adverse effects on the breastfed infant from Pretomanid Tablets or from the underlying maternal condition. When Pretomanid Tablets are administered in combination with bedaquiline and linezolid, information on lactation for bedaquiline and linezolid also applies to this combination regimen. Refer to the bedaquiline and linezolid prescribing information for more information on their use during lactation.
Data
Animal Data
In a pre- and postnatal development study in rats treated with pretomanid at doses 0.5 and 2 times the human exposure for a 200 mg dose (AUC) from gestational day 7 through lactation day 20, concentrations in milk on lactation day 14 were 1.4 and 1.6 times higher than the maximum concentration observed in maternal plasma, respectively. The concentration of pretomanid in rat milk does not necessarily predict the concentration of pretomanid in human milk.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Reduced fertility and/or testicular toxicity were observed in male rats and mice treated with oral pretomanid. These effects were associated with hormonal changes including decreased serum inhibin B and increased serum follicle stimulating hormone and luteinizing hormone in rodents [see Nonclinical Toxicology (13.1)].
Reduced fertility and testicular toxicity cannot be definitively ruled out in male human subjects at this time.
8.4 Pediatric Use
Safety and effectiveness of Pretomanid Tablets in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
The effect of hepatic impairment on the safety, effectiveness, and pharmacokinetics of pretomanid is not known.
Close8.7 Renal Impairment
The effect of renal impairment on the safety, effectiveness, and pharmacokinetics of pretomanid is not known.
-
10 OVERDOSAGE There is no experience with the treatment of acute overdose with pretomanid. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in ...
There is no experience with the treatment of acute overdose with pretomanid. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in case of deliberate or accidental overdose.
Close -
11 DESCRIPTION Pretomanid is an oral nitroimidazooxazine antimycobacterial drug. Pretomanid is a white to off-white to yellow-colored powder. The chemical name for pretomanid is ...
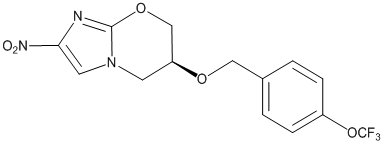
Pretomanid is an oral nitroimidazooxazine antimycobacterial drug.
Pretomanid is a white to off-white to yellow-colored powder. The chemical name for pretomanid is (6S)-2-Nitro-6-{[4-(trifluoromethoxy)phenyl]methoxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine. The molecular formula for pretomanid is C14H12F3N3O5, and the molecular weight is 359.26. The structural formula of pretomanid is as follows:
Each Pretomanid Tablet contains 200 mg of pretomanid. The inactive ingredients are colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and sodium starch glycolate.
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Pretomanid is a nitroimidazooxazine antimycobacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Cardiac Electrophysiology - A randomized ...
12.1 Mechanism of Action
Pretomanid is a nitroimidazooxazine antimycobacterial drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
A randomized, double-blind, placebo- and positive-controlled (moxifloxacin 400 mg), crossover, thorough QT study of pretomanid was performed in 74 healthy adult subjects. At 400 mg (2 times the approved recommended dosage) and 1,000 mg (5 times the approved recommended dosage) single doses of pretomanid, no significant QT prolongation effect was detected.
In Trial 1, patients received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 26 weeks. No patient had QTcF intervals greater than 480 msec, and 1 subject had a post-baseline increase of QTcF of greater than 60 msec following the combination regimen of 200 mg pretomanid once daily, 400 mg bedaquiline once daily for 2 weeks followed by 200 mg bedaquiline 3 times per week thereafter, and 1,200 mg linezolid daily.
In Trial 2, in patients who received the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 26 weeks, no patient had QTcF intervals greater than 480 msec or post-baseline increase of QTcF of greater than 60 msec following the combination regimen of 200 mg pretomanid once daily, 200 mg bedaquiline once daily for 8 weeks followed by 100 mg bedaquiline once daily thereafter, and 600 mg or 1,200 mg linezolid daily.
12.3 Pharmacokinetics
Pretomanid AUC and Cmax were approximately dose proportional over a range of single oral doses from 50 mg (0.25 times the approved recommended dosage) to 200 mg (approved recommended dosage); at single doses greater than 200 mg and up to 1,000 mg (5 times the approved recommended dosage), AUC and Cmax increased in a less than dose proportional manner. Steady-state pretomanid plasma concentrations were achieved approximately 4 to 6 days following multiple dose administration of 200 mg, and the accumulation ratio was approximately 2. Pharmacokinetic parameters following single and multiple 200 mg doses of pretomanid in healthy adult subjects are summarized in Table 3.
Table 3: Mean (SD) Pretomanid Pharmacokinetic Parameters in Healthy Adult Subjects Under Fasted and Fed Conditions ND - Not Determined. PK Parameter
Single Dose
200 mg; FastedSingle Dose
200 mg; FedSteady State
200 mg QD; FastedCmax (µg/mL)
1.1 (0.2)
2.0 (0.3)
1.7 (0.3)
AUCt (µg•hr/mL)
*28.1 (8.0)
*51.6 (10.1)
†30.2 (3.7)
AUCinf (µg•hr/mL)
28.8 (8.3)
53.0 (10.6)
ND
‡Tmax (hr)
4.0 (2.0, 6.0)
5.0 (3.0, 8.1)
4.5 (2.0, 8.0)
Vd/F (L)
180 (51.3)
97.0 (17.2)
ND
CL/F (L/hr)
7.6 (2.5)
3.9 (0.8)
ND
t½ (hr)
16.9 (3.1)
17.4 (2.8)
16.0 (1.6)
Absorption
Effect of Food
Administration of an oral tablet dose of pretomanid with a high-fat, high-calorie meal (approximately 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively) increased mean Cmax by 76% and mean AUCinf by 88% as compared with the fasted state (see also Table 3 above).
Elimination
See Table 3 above for estimates of apparent oral clearance and half-life of pretomanid.
Specific Populations
No clinically significant differences in the pharmacokinetics of pretomanid were observed based on sex, body weight, race (Black, White, or other), pulmonary TB status (resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug OR resistant to isoniazid and rifampin and treatment intolerant or non-responsive to standard therapy), or HIV status. The effect of renal or hepatic impairment on the pharmacokinetics of pretomanid is unknown.
Drug Interaction Studies
Clinical Studies
Efavirenz: Co-administration of 200 mg QD of pretomanid with efavirenz 600 mg QD for 7 days resulted in a decrease of pretomanid mean AUC by 35% and Cmax by 28%. Mean AUC and Cmax of efavirenz were not affected when given with pretomanid.
Lopinavir/Ritonavir: Co-administration of 200 mg QD pretomanid with lopinavir/ritonavir 400/100 mg BID for 7 days resulted in a decrease of pretomanid mean AUC by 17% and Cmax by 13%. Mean AUC and Cmax of lopinavir were decreased by 14% and 17%, respectively, when given with pretomanid.
Rifampin: Co-administration of 200 mg QD pretomanid with rifampin 600 mg QD for 7 days resulted in a decrease of pretomanid mean AUC by 66% and Cmax by 53%.
Midazolam: Co-administration of 400 mg (twice the approved recommended dosage) QD pretomanid for 14 days and a single 2 mg oral dose of midazolam on Day 14 resulted in a decrease in midazolam mean AUC by 15% and Cmax by 16%, and an increase in 1-hydroxy midazolam mean AUC by 14% and Cmax by 5%.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
Cytochrome P450 (CYP) Enzymes: CYP3A4 plays a role in the metabolism of pretomanid, i.e., up to 20%. Pretomanid is not a substrate of CYP2C9, CYP2C19, and CYP2D6. Pretomanid is not an inhibitor of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 at clinically relevant concentrations based on in vitro studies. Pretomanid is not an inducer of CYP3A4.
Transporter Systems: Pretomanid is an inhibitor of the OAT3 transporter in vitro, which could result in increased concentrations of OAT3 substrate medicinal products clinically and may increase the risk of adverse reactions associated with these medicines. No clinical drug-drug interaction studies have been conducted with OAT3 substrates [see Drug Interactions (7.2)].
In vitro studies cannot exclude the possibility that pretomanid is an inhibitor of BCRP, OATP1B3, and P-gp transporters. The effect of co-administration of pretomanid on the pharmacokinetics of BCRP, OATP1B3 and P-gp substrates in humans is unknown [see Drug Interactions (7.2)].
In vitro studies indicated that pretomanid does not inhibit human OAT1, OCT1, OCT2, OAT1B1, BSEP, MATE1, and/or MATE2-K mediated transport at clinically relevant concentrations of pretomanid. Pretomanid is not a substrate of OAT1, OAT3, OCT2, OAT1B1, OATP1B3, MATE1, MATE2-K, BCRP, and/or P-gp transporters.
Close12.4 Microbiology
Mechanism of Action
Pretomanid Tablet is a nitroimidazooxazine antimycobacterial drug. Pretomanid kills actively replicating M. tuberculosis by inhibiting mycolic acid biosynthesis, thereby blocking cell wall production. Under anaerobic conditions, against non-replicating bacteria, pretomanid acts as a respiratory poison following nitric oxide release. All of these activities require nitro-reduction of pretomanid within the mycobacterial cell by the deazaflavin-dependent nitroreductase, Ddn, which is dependent on the reduced form of the cofactor F420. Reduction of F420 is accomplished by the F420-dependent glucose-6-phosphate dehydrogenase, Fgd1.
Resistance
Mutations in five M. tuberculosis genes (ddn, fgd1, fbiA, fbiB, and fbiC) have been associated with pretomanid resistance. The products of these genes are involved in bioreductive activation of pretomanid within the bacterial cell. Not all isolates with increased minimum inhibitory concentrations (MICs) have mutations in these genes, suggesting the existence of at least one other mechanism of resistance. The in vitro frequency of resistance development to pretomanid ranged from 10-7 to 10-5 at 2 to 6 times the pretomanid MICs. Cross-resistance of pretomanid with other compounds in the same class has been observed.
Antimicrobial Activity
Pretomanid has demonstrated in vitro activity against the M. tuberculosis complex. Pretomanid has also demonstrated anti-M. tuberculosis activity in animal models of tuberculosis [see Indications and Usage (1)].
In murine tuberculosis models, the 3-drug combination of pretomanid, bedaquiline, and linezolid reduced bacterial counts in the lungs to a greater extent and resulted in fewer relapses at 2 and 3 months post-therapy compared to 2-drug combinations of pretomanid, bedaquiline, and linezolid.
In Trial 1, the pretomanid MIC was determined using the Mycobacterial Growth Indicator Tube (MGIT). The baseline pretomanid MIC for M. tuberculosis isolates in the trial ranged from 0.06 to 1 mcg/mL.
In Trial 2, the pretomanid MIC increased from 1 mcg/mL to 16 mcg/mL in seven patients with paired baseline and post 16 weeks isolates. Five patients (two in the linezolid 1,200 mg for 9 weeks arm, three in the linezolid 600 mg for 9 weeks arm) relapsed or failed treatment and one had a favorable outcome. The 9-week dosing regimen is not an approved dosing regimen. Six of the seven patients had elevations in bedaquiline MICs at baseline.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No mutagenic or clastogenic effects were detected in conventional genotoxicity studies with pretomanid. A ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No mutagenic or clastogenic effects were detected in conventional genotoxicity studies with pretomanid. A circulating metabolite of pretomanid, M50, was mutagenic in a bacterial reverse mutation assay. No carcinogenic potential was revealed in a 6-month study in transgenic mice where this metabolite was produced. In a 2-year study in rats, there was no evidence of carcinogenic risk.
Mutagenesis
No mutagenic or clastogenic effects were detected in both an in vitro bacterial reverse mutation assay and an in vitro mammalian chromosome aberrations assay using a Chinese hamster ovary cell line. Pretomanid was negative for clastogenicity in a mouse bone marrow micronucleus assay.
A metabolite of pretomanid was mutagenic in a bacterial reverse mutation assay. This metabolite represents approximately 6% of the human exposure (AUC) to pretomanid at the MRHD.
Fertility
In a fertility and general reproduction study in rats, male rats treated orally with pretomanid for 13 to 14 weeks had reduced fertility at 30 mg/kg/day and complete infertility at 100 mg/kg/day (approximately 1 and 2‑times the human exposure for a 200 mg dose, respectively). At 100 mg/kg/day, males had testicular atrophy including hypospermia in the epididymal tubules and focal epithelial hyperplasia of the epididymal tubular epithelium. Following a 10-week treatment-free period, these effects were partially reversed in male rats given pretomanid at 30 mg/kg/day but not at 100 mg/kg/day. These effects were associated with increased serum follicle-stimulating hormone and decreased serum inhibin B concentrations. There were no effects of pretomanid in male rats treated for 13 weeks at 10 mg/kg/day (approximately half of the human exposure for a 200 mg dose). Pretomanid did not affect mating behavior in female rats given oral pretomanid at 100 mg/kg/day for two weeks (approximately twice the human exposure).
Testicular toxicity was present in male mice treated orally for 13 weeks at 20 mg/kg/day [approximately equal to the human exposure (AUC) for a 200 mg dose]. There were no adverse testicular effects observed in mice given pretomanid at 6 mg/kg/day (0.2‑times the human exposure for a 200 mg dose).
Testicular toxicity was observed in male rats following 7 or 14 days of dosing with oral pretomanid at 100 mg/kg/day (approximately 2-times the human exposure for a 200 mg dose). The effects were partially reversible during a 6-month post treatment recovery period in rats treated with pretomanid for 7 days, but not 14 days.
In a 3-month study, decreased sperm motility and total sperm count, and increased abnormal sperm ratio were noted in sexually mature monkeys given ≥150 mg/kg/day (approximately 3 times the human exposure for a 200 mg dose).
Close13.2 Animal Toxicology and/or Pharmacology
Cataracts were observed in rats treated with pretomanid at doses of 300 mg/kg/day for 13 weeks or 100 mg/kg/day for 26 weeks. There were no cataracts observed in rats given oral pretomanid at 30 mg/kg/day (approximately 2 times the human exposure for a 200 mg dose) for 26 weeks.
In monkeys given oral pretomanid at 450 mg/kg/day for 4 weeks and 300 mg/kg/day for 12 more weeks, cataracts were not present at the end of dosing but developed during the 13‑week post treatment recovery period. In a subsequent study, cataracts were not observed following 13 weeks treatment with up to 300 mg/kg/day oral pretomanid or during the 20-week post treatment recovery period. Further, no cataracts were observed in monkeys given oral pretomanid at 100 mg/kg/day for 39 weeks with a 12-week post treatment recovery. This is approximately 1- to 2-times the human exposure (AUC) for a 200 mg dose.
-
14 CLINICAL STUDIES Trial 1 - Trial 1 (NCT02333799) was an open-label trial conducted in three centers in South Africa in patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second ...
Trial 1
Trial 1 (NCT02333799) was an open-label trial conducted in three centers in South Africa in patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug (Population 1), or pulmonary TB resistant to isoniazid and rifampin, who were treatment-intolerant or non-responsive to standard therapy (Population 2). Fifty-six (51%) patients were HIV-positive. The patients received a combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for 26 weeks (extended to 9 months in 2 patients) with 24 months of follow-up. The dosage of Pretomanid Tablets was 200 mg orally once daily. The dosage of bedaquiline was 400 mg orally once daily for 2 weeks followed by 200 mg 3 times per week for 24 weeks. The starting dose of linezolid was either 600 mg orally twice daily or 1,200 mg orally once daily. One hundred seven of the 109 patients enrolled were assessable for the primary efficacy analyses with two patients remaining in follow-up for the primary outcome assessment.
Treatment failure was defined as the incidence of bacteriologic failure (reinfection – culture conversion to positive status with a different M. tuberculosis strain), bacteriological relapse (culture conversion to positive status with the same M. tuberculosis strain), or clinical failure (an unfavorable status at, or before, end of treatment (EOT) or failing to attain culture negativity, or if the patient was withdrawn at or before EOT for clinical reasons including retreatment or changing treatment) through follow-up until 6 months after the EOT. Results are presented in Table 4. Of the 107 patients assessed, outcomes were classified as success for 95 (89%) patients and failure for 12 (11%) patients. The success rate significantly exceeded the historical success rates for TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug based on a literature review. The outcomes were similar in both HIV negative and HIV positive patients.
Table 4: Outcomes Six Months After the End of Treatment in Population 1 and Population 2 in Trial 1 Outcome
Total
Population 1*
Population 2†
Total assessable
107
71
36
Success
Success (culture negative status at 6 months post treatment)
95 (89%)
63 (89%)
32 (89%)
Failure
Death
7
6
1
Relapse post treatment
2
1‡
1
Withdrawal, loss to follow-up, or contaminated cultures
3
1
2
Total Failure
12 (11%)
8 (11%)
4 (11%)
Trial 2
Trial 2 (NCT03086486) was a phase 3 partially blinded, randomized trial assessing the safety and efficacy of various doses and treatment durations of linezolid plus Pretomanid Tablets and bedaquiline in patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug, or pulmonary TB resistant to rifamycins and either a fluoroquinolone or a second line injectable antibacterial drug, or pulmonary TB resistant to isoniazid and rifampin who were treatment intolerant or non-responsive to standard therapy.
A total of 181 patients were randomized to receive one of 4 treatment regimens, of which 45 each received 1,200 mg or 600 mg linezolid orally plus Pretomanid Tablets and bedaquiline for 26 weeks, and 46 and 45 patients received 1,200 mg or 600 mg linezolid orally for 9 weeks (not an approved dosing regimen), followed by linezolid placebo for 17 weeks, respectively, plus Pretomanid Tablets and bedaquiline for 26 weeks. The dosage of Pretomanid Tablets was 200 mg orally once daily for 26 weeks. The dosage of bedaquiline was 200 mg daily for 8 weeks, followed by 100 mg daily for 18 weeks.
Treatment failure was defined as the incidence of bacteriologic failure, bacteriologic relapse or clinical failure through follow-up until 6 months after the end of treatment, which was identical to the definition of treatment failure in Trial 1.
The success rate in each treatment arm significantly exceeded the historical success rates for TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug based on a literature review used in Trial 1. When used in combination with Pretomanid Tablets and bedaquiline, linezolid 600 mg once daily for 26 weeks is preferred over linezolid 1,200 mg once daily for 26 weeks [see Dosage and Administration (2.2)].
Among the 90 patients receiving linezolid for 26 weeks, the mean age of the patients was 38 years old with 68% being males. Most patients were White (64%), and the remaining patients were Black (36%). Most patients had a current diagnosis (a stratification factor) of pulmonary TB resistant to rifamycins and either a fluoroquinolone or a second line injectable antibacterial drug (46%) or pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug (44%), and the remainder were patients having pulmonary TB resistant to isoniazid and rifampin who were treatment intolerant or non-responsive to standard therapy (6% and 4%, respectively). The results are presented in Table 5 below.
Table 5: Outcomes Six Months After the End of Treatment* for All Randomized Patients†‡ in 26-Week Regimens in Trial 2: N = total number of patients in the relevant analysis population; n = number of patients in each category. - *
- Linezolid was administered with Pretomanid Tablets and bedaquiline.
- †
- The primary efficacy analysis excluded one randomized subject who was lost to follow-up after repeated negative cultures during the follow-up period.
- ‡
- Refers to patients with pulmonary TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug or patients with pulmonary TB resistant to isoniazid and rifampin, and who are treatment-intolerant or nonresponsive to standard therapy.
- §
- When used in combination with Pretomanid Tablets and bedaquiline, linezolid 600 mg once daily for 26 weeks is preferred over linezolid 1,200 mg once daily for 26 weeks based on safety profiles [see Dosage and Administration (2.2) and Adverse Reactions (6.1)].
Outcome
Linezolid
1,200 mg§26 weeks
(N = 44†)
n (%)
Linezolid
600 mg§
26 weeks
(N = 45)
n (%)
Success
41 (93%)
41 (91%)
Failure
Relapse post treatment
0
1 (2%)
Re-treated post treatment
2 (5%)
1 (2%)
Loss to follow-up or withdrawal
1 (2%)
2 (4%)
Total Failure
3 (7%)
4 (9%)
Close -
16 HOW SUPPLIED/STORAGE AND HANDLING Pretomanid Tablet 200 mg is packaged in either white, round, high-density polyethylene bottles with polypropylene child-resistant closure or child-resistant blister packages comprised of a ...
Pretomanid Tablet 200 mg is packaged in either white, round, high-density polyethylene bottles with polypropylene child-resistant closure or child-resistant blister packages comprised of a polyvinylchloride film with foil and paper backing.
Pretomanid Tablet 200 mg is a white to off-white, oval tablet debossed with M on one side and P200 on the other side.
NDC Number
Size
49502-476-26
Bottle of 26
49502-476-14
Unit dose blister pack of 14 (1 strip of 14 tablets)
49502-476-29
Unit dose blister pack of 100 (10 strips of 10 tablets)
49502-476-72
Bottle of 182
Store below 30°C (86°F).
Dispense only in original container and keep container tightly closed.
Close -
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Medication Guide). Important Information on Co-administration of Pretomanid Tablets in Combination with Bedaquiline and Linezolid ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Important Information on Co-administration of Pretomanid Tablets in Combination with Bedaquiline and Linezolid [see Dosage and Administration (2.1)]
- •
- Inform the patient or caregiver that Pretomanid Tablets administered as a combination regimen with bedaquiline and linezolid would be useful only in adult patients with TB resistant to isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibacterial drug or TB resistant to isoniazid and rifampin, who are treatment-intolerant or nonresponsive to standard therapy. This regimen is not indicated for treatment in patients with latent infection or extra-pulmonary infection due to M. tuberculosis, drug-sensitive TB, TB resistant to isoniazid and rifampin who are responsive to standard therapy and not treatment-intolerant, or who have TB with known resistance to any component of the regimen (Pretomanid Tablets, bedaquiline, or linezolid).
- •
- Instruct the patient or caregiver that the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid must be administered by directly observed therapy (DOT).
- •
- Inform patients of safety concerns associated with linezolid and bedaquiline and advise the patient or their caregiver to read the Medication Guide for bedaquiline.
Serious Adverse Reactions
Advise patients that the following serious adverse reactions can occur with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid: liver enzyme abnormalities, myelosuppression including anemia, peripheral and optic neuropathy, and cardiac rhythm abnormalities [see Warnings and Precautions (5.2, 5.4)].
Additional serious adverse reactions can occur with the use of linezolid, including serotonin syndrome, lactic acidosis, and convulsions. Refer to the prescribing information for linezolid for additional counseling information for these serious adverse reactions.
Peripheral and Optic Neuropathy
Advise patients to promptly inform their physician if they experience changes in vision during linezolid therapy. Monitor visual function in all patients receiving linezolid. Counsel patients to obtain prompt ophthalmological evaluation if the patient experiences symptoms of visual impairment [see Warnings and Precautions (5.3)].
Interruption of Linezolid Dosing to Manage Linezolid Adverse Reactions
Counsel patients that linezolid dosing may be modified or interrupted during the therapy to manage the known linezolid adverse reactions of myelosuppression, peripheral neuropathy, and optic neuropathy [see Dosage and Administration section (2.2) and Adverse Reactions (6)].
Compliance with Treatment
Inform patients that Pretomanid Tablets must be taken as part of a combination regimen with bedaquiline and linezolid. Compliance with the full course of therapy must be emphasized. Advise patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed for the full prescribed duration of dosing. Skipping doses other than as directed by a physician or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that their Mycobacterium may develop resistance and the disease will not be treatable by the regimen or other antibacterial drugs in the future.
Important Administration Instructions
- •
- Inform patients to take the regimen with food. Doses of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid missed for safety reasons can be made up at the end of treatment; doses of linezolid alone missed due to linezolid adverse reactions should not be made up. If bedaquiline and/or Pretomanid Tablets are permanently discontinued, the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid should be discontinued.
- •
- Advise patients who have difficulty swallowing tablets that Pretomanid Tablets can be crushed and suspended in water at room temperature. Alternately, the tablet can be soaked for 4 to 5 minutes in room temperature water and then the remaining solid crushed [see Dosage and Administration (2.2)].
Use in Patients with Hepatic or Renal Impairment
Advise patients to inform their physician if they have a history of liver or kidney problems. The safety and effectiveness of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid in populations with hepatic or renal impairment have not been established.
Use with Alcohol and Other Medications
Advise patients to discuss with their physician the other medications they are taking and other medical conditions before starting treatment with Pretomanid Tablets.
Advise patients to abstain from alcohol, hepatotoxic medications, and herbal products [see Drug Interactions (7)].
Manufactured by:
Mylan Laboratories Limited
Hyderabad, 500 096, IndiaManufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Under license from TB Alliance.MS:MXA:PRET:R4
Close -
MEDICATION GUIDE Pretomanid Tablets (Pre-TOH-mah-nid) Limited Population - What is the most important information I should know about the combination regimen of Pretomanid Tablets, bedaquiline, and ...
Pretomanid Tablets (Pre-TOH-mah-nid) Limited Population
What is the most important information I should know about the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
Pretomanid Tablets are for use only as part of a combination antibiotic regimen that includes Pretomanid Tablets, bedaquiline, and linezolid.
Treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause serious side effects. See “What are the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?”
Read the Medication Guide that comes with bedaquiline. Ask your healthcare provider for information about linezolid. The serious side effects that can happen when taking bedaquiline and linezolid can also happen when taken in the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
What is the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
Pretomanid Tablets are a prescription medicine used as part of a combination regimen with bedaquiline and linezolid. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid includes three prescription antibiotics that are used together in adults to treat tuberculosis (TB) of the lungs that is resistant to other classes of antibiotics (isoniazid, rifamycins, a fluoroquinolone and a second line injectable antibiotic) or in adults who cannot tolerate or do not respond to treatment for TB that is resistant to two specific antibiotics (isoniazid and rifampin).
Pretomanid Tablets are not for use in people who have:
- •
- TB that is not resistant to antibiotics.
- •
- an inactive (latent) TB infection.
- •
- a type of TB other than TB of the lungs.
- •
- TB resistant to isoniazid and rifampin who can tolerate or who respond to medicines usually used to treat this type of TB.
- •
- TB that is known to be resistant to Pretomanid Tablets, bedaquiline, or linezolid.
It is not known if Pretomanid Tablets are safe and effective for use except in combination with bedaquiline and linezolid as part of the recommended dosing regimen.
It is not known if Pretomanid Tablets are safe and effective in children.
Pretomanid Tablets were approved by FDA using the Limited Population pathway. This means FDA has approved this drug for a limited and specific patient population, and studies on the drug may have only answered focused questions about its safety and effectiveness.
Do not take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if:
- •
- you have been told by your healthcare provider not to take bedaquiline or linezolid.
Before you take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have liver problems. See “What are the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?”
- •
- have kidney problems.
- •
- have or have had an abnormal heart rhythm (ECG) or other heart problems, including heart failure.
- •
- have a family history of a heart problem called “congenital long QT syndrome.”
- •
- have decreased thyroid gland function (hypothyroidism).
- •
- have HIV infection.
- •
- have been told that you have low levels of calcium, magnesium or potassium in your blood.
- •
- have ever had a seizure.
- •
- are pregnant or plan to become pregnant. It is not known if pretomanid will harm your unborn baby. Talk to your healthcare provider about the possible risks to your baby if you take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid while you are pregnant.
- •
- are breastfeeding or plan to breastfeed. It is not known if pretomanid passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. You should not take herbal products or medicines that can harm your liver during treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid regimen may affect how other medicines work, and other medicines may affect how the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid works.
Especially tell your healthcare provider if you take:
- •
- a type of medicine called a CYP3A4 inducer, such as efavirenz or rifampin. Ask your healthcare provider if you are not sure.
How should I take the combination regimen of Pretomanid Tablets, bedaquiline and linezolid?
- •
- Pretomanid Tablets must only be taken with bedaquiline and linezolid as part of the dosing regimen prescribed by your healthcare provider.
- •
- Take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid exactly as your healthcare provider tells you to take it.
- •
- It is important that you complete the full course of treatment with the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, and not miss any doses, even if you feel better before you have completed the full course of treatment. Missing doses may decrease the effectiveness of the treatment and increase the chance that your TB will not be treatable by the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid or other medicines.
- •
- Take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for a total of 26 weeks.
- o
- Your healthcare provider will tell you if you should take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for longer than 26 weeks.
- o
- Your healthcare provider will tell you if you should stop taking linezolid before you have taken it for 26 weeks or if you should reduce your dose of linezolid due to side effects.
- •
- Your healthcare provider or caregiver will watch you take your doses of Pretomanid Tablets, bedaquiline, and linezolid.
- •
- Take Pretomanid Tablets, bedaquiline, and linezolid with food.
- o
-
If you can swallow whole tablets:
- ▪
- Swallow tablets whole with water.
- o
-
If you cannot swallow whole tablets:
- ▪
- Crush 200 mg of Pretomanid Tablets (1 tablet) and mix with 1 teaspoon (5 mL) of room temperature drinking water. Mix well in a drinking cup.
- ▪
- Swallow mixture immediately.
- ▪
- Make sure no remaining medicine is left in the drinking cup. Rinse well with another 1 teaspoon (5 mL) of room temperature drinking water and swallow the mixture immediately.
- ▪
- Do not store mixture for later use. Or
- ▪
- Soak 200 mg of Pretomanid Tablets (1 tablet) in 1 teaspoon (5 mL) of room temperature drinking water for 4 to 5 minutes in a drinking cup. Then crush the tablet in the drinking cup. Mix the medicine in the drinking cup well by stirring.
- ▪
- Swallow the mixture immediately.
- ▪
- Make sure no remaining medicine is left in the drinking cup. Rinse well with another 1 teaspoon (5 mL) of room temperature drinking water and swallow the mixture immediately.
- ▪
- Do not store the mixture for later use.
- •
-
Week 1 and Week 2:
- o
- Take 200 mg of Pretomanid Tablets (1 tablet), 400 mg of bedaquiline, and 600 mg of linezolid 1 time each day. Or
- o
- Take 200 mg of Pretomanid Tablets (1 tablet), 400 mg of bedaquiline, and 1,200 mg of linezolid 1 time each day.
- •
-
Week 3 to Week 26:
- o
- Take 200 mg of Pretomanid Tablets (1 tablet) and 600 mg of linezolid 1 time each day. Or
- o
- Take 200 mg of Pretomanid Tablets (1 tablet) and 1,200 mg of linezolid 1 time each day.
- o
- Take 200 mg of bedaquiline 3 times a week.
- ▪
- Take your doses of bedaquiline at least 48 hours apart. For example, you may take bedaquiline on Monday, Wednesday, and Friday every week from Week 3 to Week 26. Or
- •
-
Week 1 to Week 8:
- o
- Take 200 mg of bedaquiline 1 time each day for 8 weeks. Then take 100 mg of bedaquiline 1 time each day for 18 weeks, for a total of 26 weeks.
- •
- You may need to take the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for longer than 26 weeks. Talk with your healthcare provider.
- •
- Do not miss a dose of Pretomanid Tablets, bedaquiline, or linezolid unless instructed to do so by your healthcare provider. If you miss doses or do not complete the total 26 weeks of treatment, your treatment may not work as well, and your TB may be harder to treat.
- •
- If your healthcare provider tells you to stop taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid for a period of time, follow the instructions given to you by your healthcare provider for taking the missed doses at the end of your treatment. You should not make up any missed doses of linezolid alone that your healthcare provider told you not to take due to side effects.
- •
- If you miss a dose of Pretomanid Tablets, bedaquiline, or linezolid and you are not sure what to do, talk to your healthcare provider as soon as possible.
- •
- If you take too much Pretomanid Tablets, go to the nearest hospital emergency room right away.
Do not stop taking Pretomanid Tablets, bedaquiline, or linezolid without first talking to your healthcare provider.
What should I avoid when taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
- •
- You should not drink alcohol while taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
What are the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid may cause serious side effects including:
- •
- See “What is the most important information I should know about the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?”
- •
- Liver problems. Your healthcare provider should do blood tests to check your liver at least before you start taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, 2 weeks after starting treatment, and then monthly during treatment. Tell your healthcare provider right away if you have symptoms of liver problems, such as:
- o
- unusual tiredness
- o
- loss of appetite
- o
- nausea
- o
- yellowing of your skin or the whites of your eyes
- o
- dark (tea-colored) urine
- o
- tenderness in the upper right side of your stomach-area (abdomen)
- See “What should I avoid when taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?”
- •
- Low blood cell counts. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause low red blood cell counts (anemia), low white blood cell counts (leukopenia), low blood platelet counts (thrombocytopenia) or a combination of low red and white blood cell counts and low blood platelet counts (pancytopenia). Low blood cell counts are a side effect of linezolid. Anemia is a common side effect of linezolid, but can be serious and life-threatening. Low blood cell counts were reversible when linezolid treatment was reduced, stopped for a period of time, or stopped permanently. Your healthcare provider should check your blood cell counts at least before you start taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid, 2 weeks after starting treatment, and then monthly during treatment. Your healthcare provider may lower the dose or stop treatment with linezolid if you develop or have worsening blood cell counts.
- •
- Nerve problems in your arms, hands, legs, and feet (peripheral neuropathy). The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause nerve problems in your arms, hands, legs, and feet. Tell your healthcare provider if you have symptoms of nerve problems in your arms, hands, legs, or feet, including:
-
- o
- numbness
- o
- burning
- o
- a feeling of “pins and needles”
- o
- tremors
- o
- problems with balance
- o
- weakness
- •
- Vision problems. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause nerve problems in your eyes (optic neuropathy). Nerve problems in your eyes can cause problems with your vision. Tell your healthcare provider right away if you have symptoms of nerve problems in your eyes, such as changes in vision.
- Nerve problems in your arms, hands, legs, feet, and eyes are common side effects of long-term use of linezolid, but can be serious. Nerve problems caused by linezolid were generally reversible or improved when symptoms of nerve problems were monitored by the healthcare provider and linezolid treatment was reduced, stopped for a period of time, or stopped permanently.
- •
- Heart rhythm problem called QT prolongation. The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause a heart rhythm problem. Heart rhythm problem is a side effect of bedaquiline. Your healthcare provider should do a test called an electrocardiogram (ECG) to check your heart before you start taking the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid and at least 2, 12, and 24 weeks after starting treatment. Tell your healthcare provider right away if you:
-
- o
- have a change in heartbeat (a fast or irregular heartbeat)
- o
- feel dizzy or faint
- •
- Effects on male fertility. It is not known if pretomanid can cause fertility problems in males. This could affect your ability to father a child. Talk to your healthcare provider if this is a concern for you.
- •
- Build-up of an acid in your blood (lactic acidosis). The combination regimen of Pretomanid Tablets, bedaquiline, and linezolid can cause a build-up of acid in your blood. A build-up of acid in your blood is a side effect of linezolid.
- •
- Call your healthcare provider right away if you have nausea and vomiting that keep coming back.
The most common side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid include:
- •
- nausea
- •
- acne
- •
- vomiting
- •
- abnormal blood tests that may be due to injury to your liver
- •
- headache
- •
- muscle and bone pain
- •
- heartburn
- •
- rash
- •
- itching
- •
- decreased appetite
- •
- stomach area (abdominal) pain
- •
- chest pain that worsens when you breathe or cough
- •
- coughing up blood
- •
- abnormal blood tests that may be due to injury to your pancreas
These are not all the possible side effects of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Pretomanid Tablets?
- •
- Store Pretomanid Tablets, bedaquiline, and linezolid below 86°F (30°C). Ask your pharmacist if you have questions about how to store bedaquiline and linezolid.
- •
- Keep Pretomanid Tablets in the original container with the container tightly closed.
Keep Pretomanid Tablets and all medicines out of the reach of children.
General information about the safe and effective use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Pretomanid Tablets, bedaquiline, or linezolid for a condition for which it was not prescribed. Do not give Pretomanid Tablets, bedaquiline, or linezolid to other people, even if they have the same symptoms that you have. This may harm them. You can ask your pharmacist or healthcare provider for information about Pretomanid Tablets, bedaquiline, and linezolid that is written for health professionals.
What are the ingredients in the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid?
Pretomanid Tablets active ingredient: pretomanid
Pretomanid Tablets inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and sodium starch glycolate
The ingredients for bedaquiline can be found in the Medication Guide for bedaquiline. The ingredients for linezolid can be found in the information about linezolid that is written for health professionals.
Manufactured by:
Mylan Laboratories Limited
Hyderabad, 500 096, IndiaManufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Under license from TB Alliance.For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
MS:MXA:MG:PRET:R4
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 11/2024
Close -
PRINCIPAL DISPLAY PANEL – 200 mg NDC 49502-476-26 - Rx only - Pretomanid - Tablets - 200 mg - Limited - Population* Dispense the enclosed - Medication Guide to - each patient. *See the full prescribing - information for Pretomanid - Tablets ...
NDC 49502-476-26
Rx only
Pretomanid
Tablets200 mg
Limited
Population*Dispense the enclosed
Medication Guide to
each patient.*See the full prescribing
information for Pretomanid
Tablets 200 mg for information
about the limited population.26 tablets
Each tablet contains:
Pretomanid 200 mgRecommended Dosage:
see Prescribing Information.Keep this and all medication
out of the reach of children.Store below 30°C (86°F).
Dispense only in original container.
Keep container tightly closed.
Manufactured by: Mylan Laboratories Limited
Hyderabad - 500 096, IndiaManufactured for: Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.Under license from TB Alliance.
Code No.: MH/DRUGS/AD/089
MS:MXA:47626:1C:R3
Mylan.com
Close -
INGREDIENTS AND APPEARANCEProduct Information
PRETOMANID pretomanid tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49502-476 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pretomanid (UNII: 2XOI31YC4N) (Pretomanid - UNII:2XOI31YC4N) Pretomanid 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) Product Characteristics Color WHITE (white to off-white) Score no score Shape OVAL Size 18mm Flavor Imprint Code M;P200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49502-476-26 1 in 1 CARTON 11/07/2019 1 26 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49502-476-14 14 in 1 CARTON 01/01/2029 2 NDC:49502-476-32 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:49502-476-72 1 in 1 CARTON 01/01/2029 3 182 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:49502-476-29 100 in 1 CARTON 01/01/2029 4 NDC:49502-476-33 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212862 11/07/2019
CloseLabeler - Viatris Specialty LLC (117455616)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
PRETOMANID tablet
Number of versions: 8
Get Label RSS Feed for this Drug
PRETOMANID tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=f1906fc9-cb3c-4e13-8a4a-da76100c1bf3
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
PRETOMANID tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 49502-476-14 |
| 2 | 49502-476-26 |
| 3 | 49502-476-29 |
| 4 | 49502-476-32 |
| 5 | 49502-476-33 |
| 6 | 49502-476-72 |