Label: PRESTALIA- perindopril arginine and amlodipine besylate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 72931-010-02, 72931-010-03, 72931-011-02, 72931-011-03, view more - Packager: Adhera Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 25, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRESTALIA® tablets safely and effectively. See full prescribing information for PRESTALIA. PRESTALIA (perindopril arginine and ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue PRESTALIA as soon as possible [see Warnings and Precautions (5.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGEPRESTALIA contains perindopril arginine, an angiotensin converting enzyme inhibitor, and amlodipine, a dihydropyridine calcium channel blocker, and is indicated for the treatment of hypertension ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - The recommended starting dose of PRESTALIA is 3.5/2.5 mg once daily. Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration ...
-
3 DOSAGE FORMS AND STRENGTHSPRESTALIA is available as fixed dose combination tablets of perindopril arginine and amlodipine: 3.5/2.5 mg tablets: white, uncoated tablets debossed with 3.5 on one side and 2.5 on the other ...
-
4 CONTRAINDICATIONSPRESTALIA tablets are contraindicated in patients with hereditary or idiopathic angioedema, with or without previous ACE inhibitor treatment, and in patients who are hypersensitive to perindopril ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Pregnancy Category D - Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSPrestalia - The pharmacokinetics of perindopril and amlodipine are not altered when the drugs are co-administered. No drug interaction studies have been conducted with PRESTALIA, although ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category D [see Warnings and Precautions (5.1)] Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces ...
-
10 OVERDOSAGEPerindopril - In animals, doses of perindopril up to 2,500 mg/kg in mice, 3,000 mg/kg in rats and 1,600 mg/kg in dogs were non-lethal. Past experiences were scant but suggested that overdosage ...
-
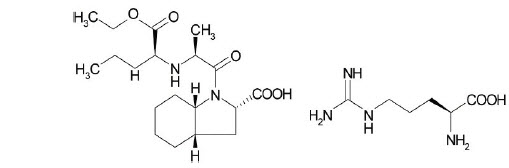
11 DESCRIPTIONPRESTALIA is a combination of perindopril arginine and amlodipine besylate. Perindopril arginine is the L-arginine salt of perindopril, the ethyl ester of a non-sulfhydryl angiotensin converting ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Perindopril - Perindopril, a pro-drug, is hydrolyzed to perindoprilat, which inhibits ACE in humans and in animals. ACE is a peptidyl dipeptidase that catalyzes the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, mutagenicity or fertility studies have been conducted with the combination of perindopril and amlodipine. However ...
-
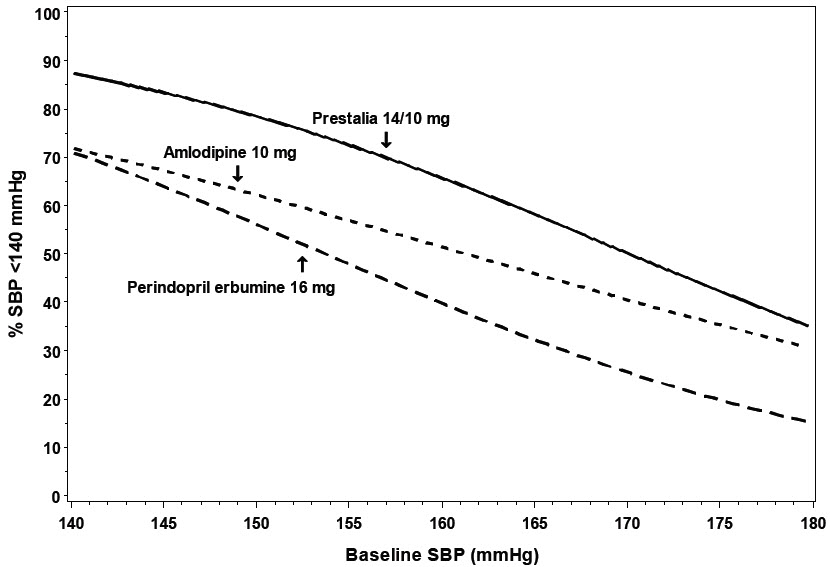
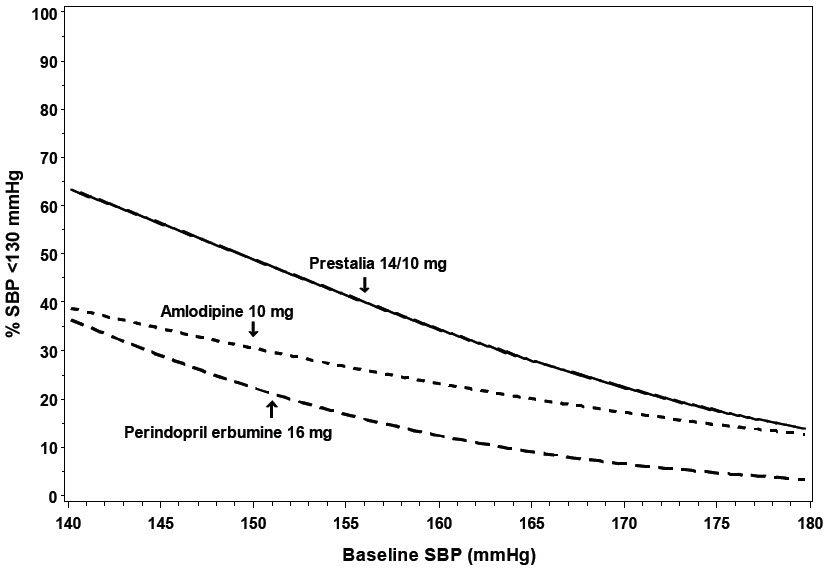
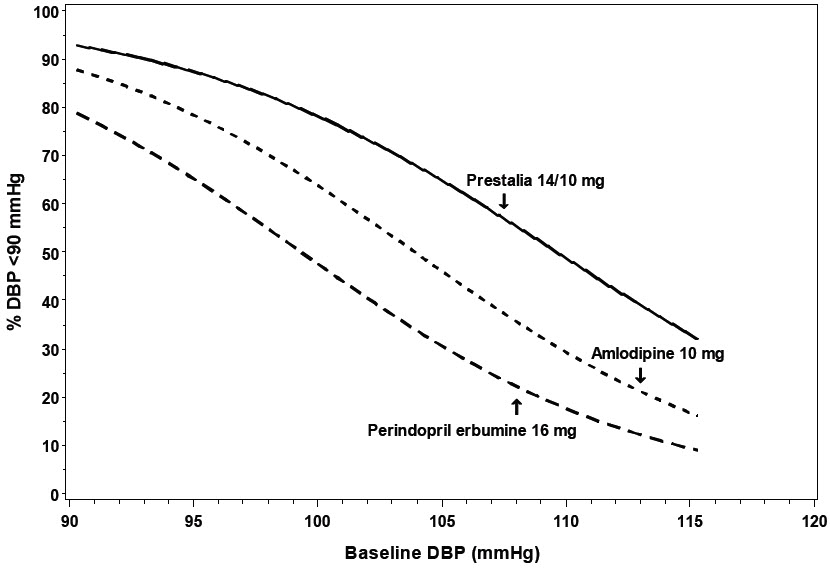
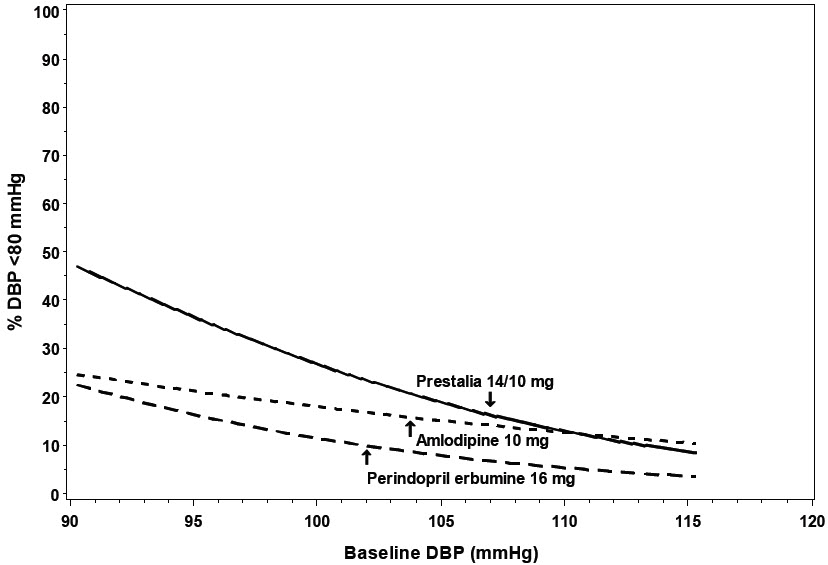
14 CLINICAL STUDIESThe antihypertensive effects of PRESTALIA have been demonstrated in two randomized controlled trials. The highest strength of PRESTALIA (14/10 mg) was studied in a double-blind, active controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPRESTALIA is available as white, uncoated tablets containing perindopril arginine 3.5 mg, 7 mg, or 14 mg and amlodipine 2.5 mg, 5 mg, or 10 mg for the following three combinations of perindopril ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Tell female patients of childbearing age that use of drugs like perindopril that act on the renin-angiotensin ...
-
Patient Information PRESTALIA® (pres-ta-li-a)(perindopril arginine and amlodipine)tabletsRead this Patient Information before you start taking PRESTALIA and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor ...
-
PRINCIPAL DISPLAY PANEL - 3.5 mg/2.5 mg Tablet Bottle Label90 Tablets - NDC 72931-010-02 - Prestalia® (perindopril arginine and - amlodipine) Tablets - 3.5 mg/2.5 mg - Rx ONLY - Keep out of reach of children - adhera - THERAPEUTICS
-
PRINCIPAL DISPLAY PANEL - 7 mg/5 mg Tablet Bottle Label90 Tablets - NDC 72931-011-02 - Prestalia® (perindopril arginine and - amlodipine) Tablets - 7 mg/5 mg - Rx ONLY - Keep out of reach of children - adhera - THERAPEUTICS
-
PRINCIPAL DISPLAY PANEL - 14 mg/10 mg Tablet Bottle Label90 Tablets - NDC 72931-012-02 - Prestalia® (perindopril arginine and - amlodipine) Tablets - 14 mg/10 mg - Rx ONLY - Keep out of reach of children - adhera - THERAPEUTICS
-
INGREDIENTS AND APPEARANCEProduct Information