Label: PREPIDIL- dinoprostone gel
- NDC Code(s): 0009-3359-01, 0009-3359-02
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Endocervical Use
-
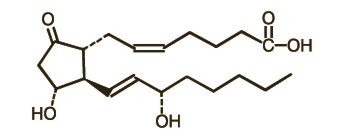
DESCRIPTIONPREPIDIL Gel contains dinoprostone as the naturally occurring form of prostaglandin E2 (PGE2) and is designated chemically as (5Z, 11a, 13E, 15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid ...
-
CLINICAL PHARMACOLOGYPREPIDIL Gel (dinoprostone) administered endocervically may stimulate the myometrium of the gravid uterus to contract in a manner similar to contractions seen in the term uterus during labor ...
-
INDICATIONS AND USAGEPREPIDIL Gel is indicated for ripening an unfavorable cervix in pregnant women at or near term with a medical or obstetrical need for labor induction.
-
CONTRAINDICATIONSEndocervically administered PREPIDIL Gel is not recommended for the following: a. Patients in whom oxytocic drugs are generally contraindicated or where prolonged contractions of the uterus are ...
-
WARNINGSFOR HOSPITAL USE ONLY - Dinoprostone, as with other potent oxytocic agents, should be used only with strict adherence to recommended dosages. Dinoprostone should be administered by physicians in a ...
-
PRECAUTIONS1. General Precautions - During use, uterine activity, fetal status, and character of the cervix (dilation and effacement) should be carefully monitored either by auscultation or electronic fetal ...
-
ADVERSE REACTIONSPREPIDIL Gel is generally well-tolerated. In controlled trials, in which 1731 women were entered, the following events were reported at an occurrence of ≥ 1%: Adverse ReactionPGE2 - (N ...
-
DRUG ABUSE AND DEPENDENCENo drug abuse or drug dependence has been seen with the use of PREPIDIL Gel.
-
OVERDOSAGEOverdosage with PREPIDIL Gel may be expressed by uterine hypercontractility and uterine hypertonus. Because of the transient nature of PGE2-induced myometrial hyperstimulation, nonspecific ...
-
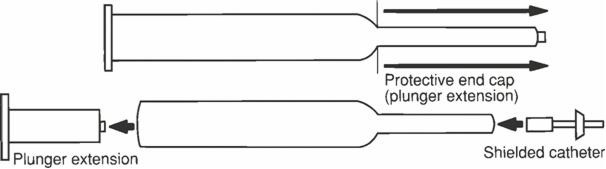
DOSAGE AND ADMINISTRATIONNOTE: USE CAUTION IN HANDLING THIS PRODUCT TO PREVENT CONTACT WITH SKIN. WASH HANDS THOROUGHLY WITH SOAP AND WATER AFTER ADMINISTRATION. PREPIDIL Gel should be brought to room temperature (59° to ...
-
HOW SUPPLIEDPREPIDIL Gel is available as a sterile semitranslucent viscous preparation for endocervical application: 0.5 mg PGE2 per 3.0 g (2.5 mL) in syringe. In addition, each package contains two shielded ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For current full prescribing information, please visit www.pfizer.com - Rx only - LAB-0062-9.0 - Revised 03/2025
-
PRINCIPAL DISPLAY PANEL - 3 g Syringe Applicator LabelNDC 0009-3359-01 - Prepidil® Gel 0.5 mg* dinoprostone cervical gel - For endocervical administration - Sterile - DOSAGE AND USE: See accompanying prescribing - information. * Each 3 gram syringe ...
-
PRINCIPAL DISPLAY PANEL - 3 g Syringe LabelPrepidil® Gel 0.5 mg - dinoprostone cervical gel - Sterile - Store in a refrigerator 2° to 8°C (36° to 46°F). One 3 gram syringe - Pfizer Injectables - Distributed by - Pharmacia & Upjohn ...
-
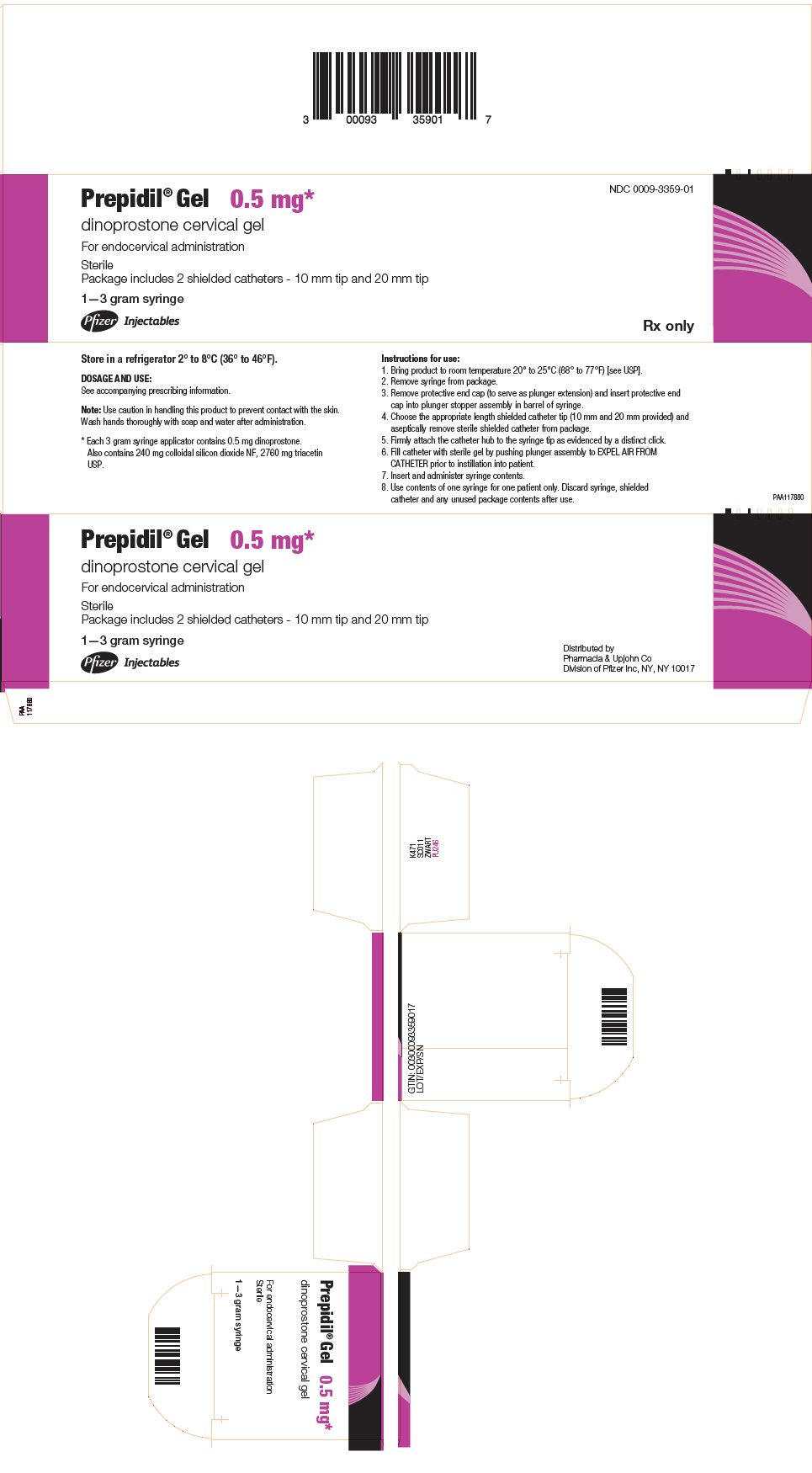
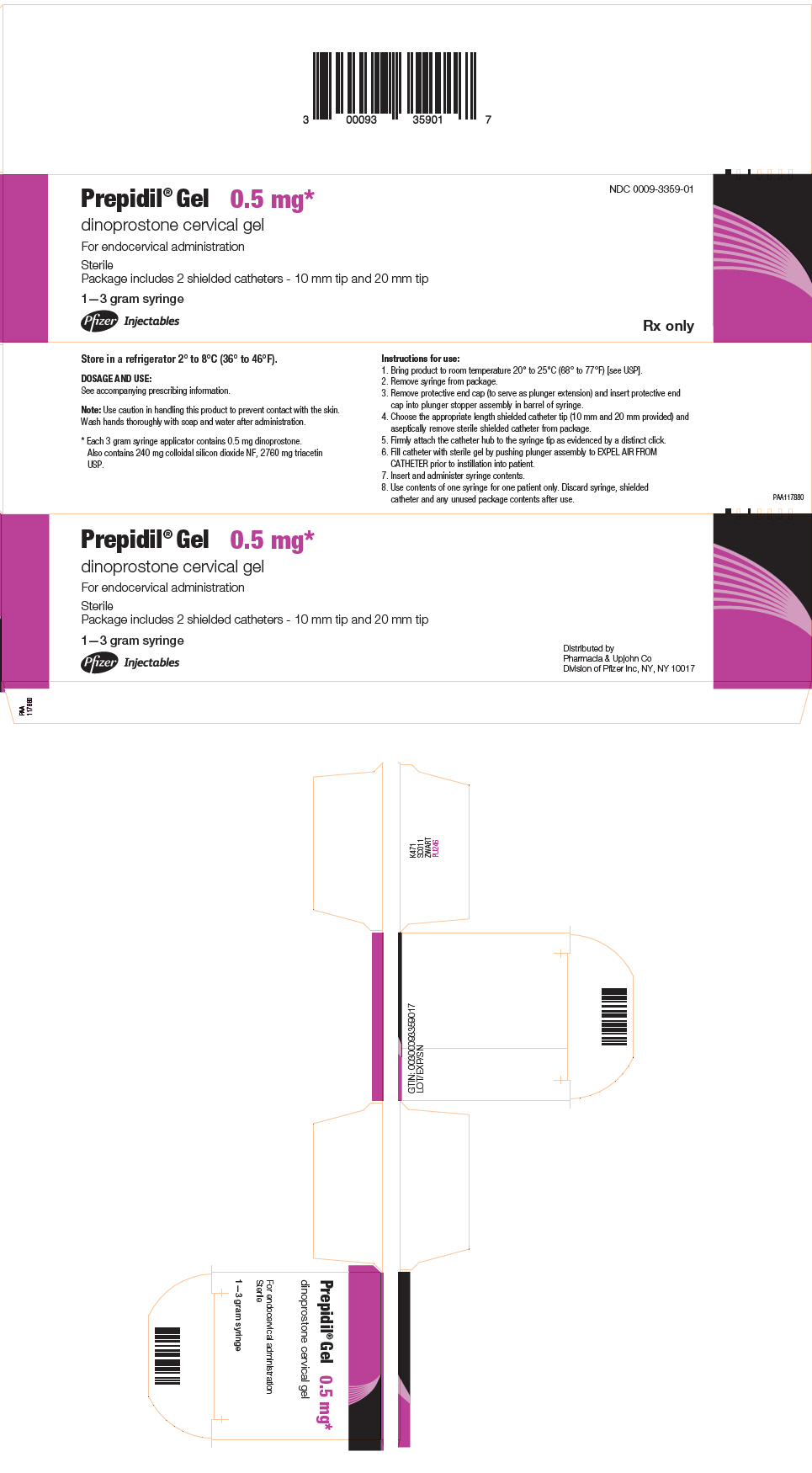
PRINCIPAL DISPLAY PANEL - 3 gram Syringe CartonPrepidil® Gel 0.5 mg* dinoprostone cervical gel - For endocervical administration - Sterile - Package includes 2 shielded catheters - 10 mm tip and 20 mm tip - 1–3 gram syringe - Pfizer ...
-
PRINCIPAL DISPLAY PANEL - 3 g Syringe Bundle LabelNDC 0009-3359-02 - Contains 5 of NDC 0009-3359-01 - Prepidil® Gel 0.5 mg - dinoprostone cervical gel - 5–3 gram syringes - Distributed by - Pharmacia & Upjohn Co - Division of Pfizer Inc, NY, NY ...
-
INGREDIENTS AND APPEARANCEProduct Information