Label: PRAXBIND- idarucizumab injection
- NDC Code(s): 0597-0197-05
- Packager: Boehringer Ingelheim Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRAXBIND safely and effectively. See full prescribing information for PRAXBIND. PRAXBIND® (idarucizumab) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPRAXBIND is indicated in patients treated with Pradaxa when reversal of the anticoagulant effects of dabigatran is needed: For emergency surgery/urgent procedures - In life-threatening or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - The recommended dose of PRAXBIND is 5 g, provided as two separate vials each containing 2.5 g/50 mL idarucizumab (see Figure 1). Both vials are packaged together in one ...
-
3 DOSAGE FORMS AND STRENGTHSPRAXBIND is a sterile, preservative-free, colorless to slightly yellow, clear to slightly opalescent solution available as: Injection: 2.5 g/50 mL solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolic Risk - Patients being treated with dabigatran therapy have underlying disease states that predispose them to thromboembolic events. Reversing dabigatran therapy exposes ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described in more detail elsewhere in the labeling: Thromboembolic Risk [see Warnings and Precautions (5.1)] Hypersensitivity Reactions [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on PRAXBIND use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. Animal reproductive and ...
-
11 DESCRIPTIONIdarucizumab is a humanized monoclonal antibody fragment (Fab) derived from an IgG1 isotype molecule, whose target is the direct thrombin inhibitor dabigatran. Using recombinant expression ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Idarucizumab is a specific reversal agent for dabigatran. It is a humanized monoclonal antibody fragment (Fab) that binds to dabigatran and its acylglucuronide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with idarucizumab. No animal studies have been performed to evaluate the ...
-
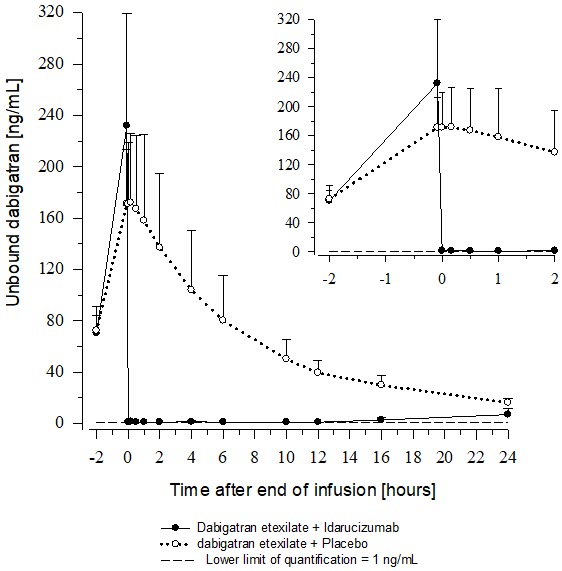
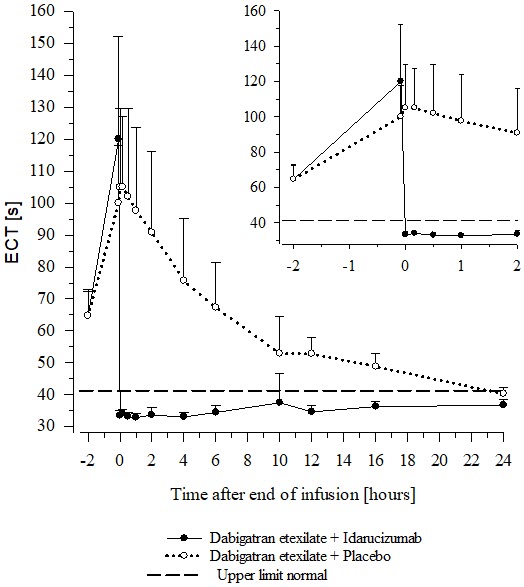
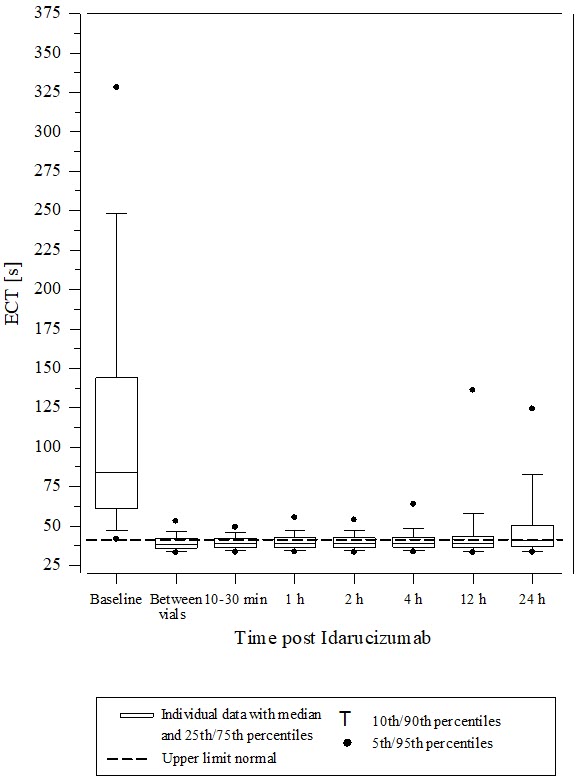
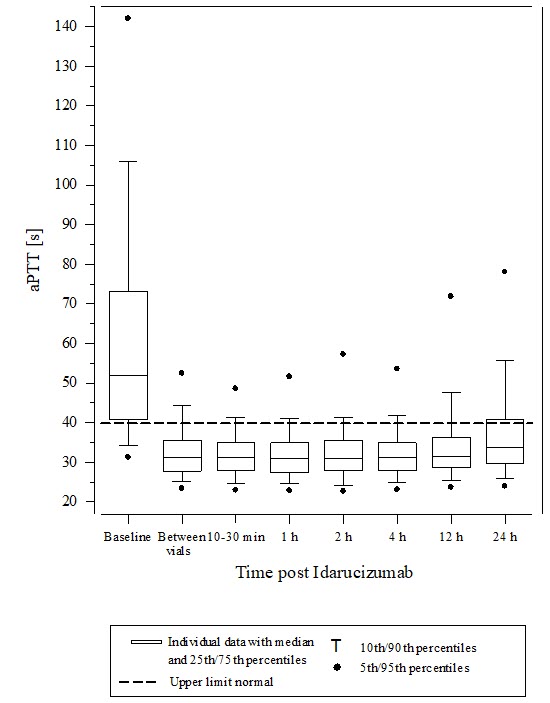
14 CLINICAL STUDIESThe safety and effectiveness of PRAXBIND was investigated in three randomized placebo-controlled healthy volunteer trials, Trials 1321.1, 1321.2 and 1321.5 (NCT01688830, NCT01955720, NCT02028780) ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - PRAXBIND is a sterile, preservative-free, colorless to slightly yellow, clear to slightly opalescent solution supplied as 2 single-dose vials each containing 2.5 g/50 mL of ...
-
17 PATIENT COUNSELING INFORMATIONThromboembolic Risk - Inform patients that reversing dabigatran therapy exposes them to the thromboembolic risk of their underlying disease. To reduce this risk, resumption of anticoagulant ...
-
SPL UNCLASSIFIED SECTIONBoehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA - US License No. 2006 - Product of Germany - Licensed from: Boehringer Ingelheim International GmbH - Copyright © 2023 Boehringer ...
-
PRINCIPAL DISPLAY PANEL - 50 mL Vial LabelNDC 0597-0197-05 - Rx only - Praxbind® (idarucizumab) Injection - 2.5 g/50 mL - (50 mg/mL) For Intravenous Use Only - Discard Unused Portion. Administer 2 vials for complete dose of 5 g - 50 mL Single-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information