Label: POTELIGEO- mogamulizumab-kpkc injection
- NDC Code(s): 42747-761-01
- Packager: Kyowa Kirin, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTELIGEO safely and effectively. See full prescribing information for POTELIGEO. POTELIGEO® (mogamulizumab-kpkc) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPOTELIGEO is indicated for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of POTELIGEO is 1 mg/kg administered as an intravenous infusion over at least 60 minutes. Administer on days 1, 8, 15, and 22 of the first 28-day ...
-
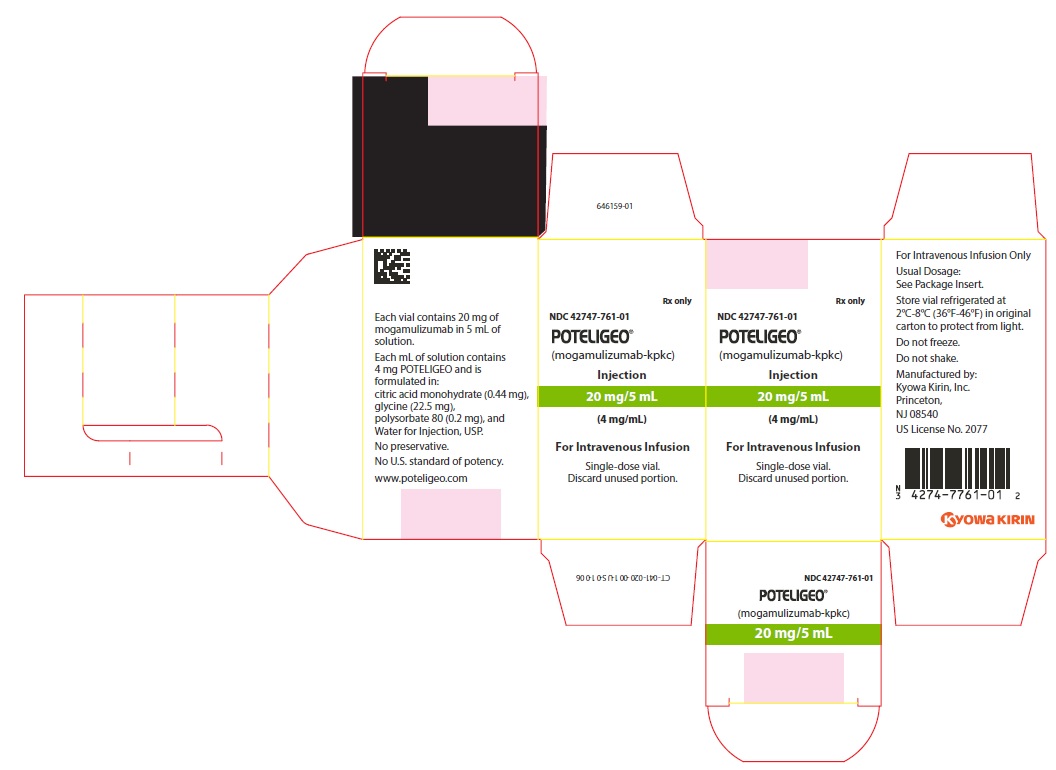
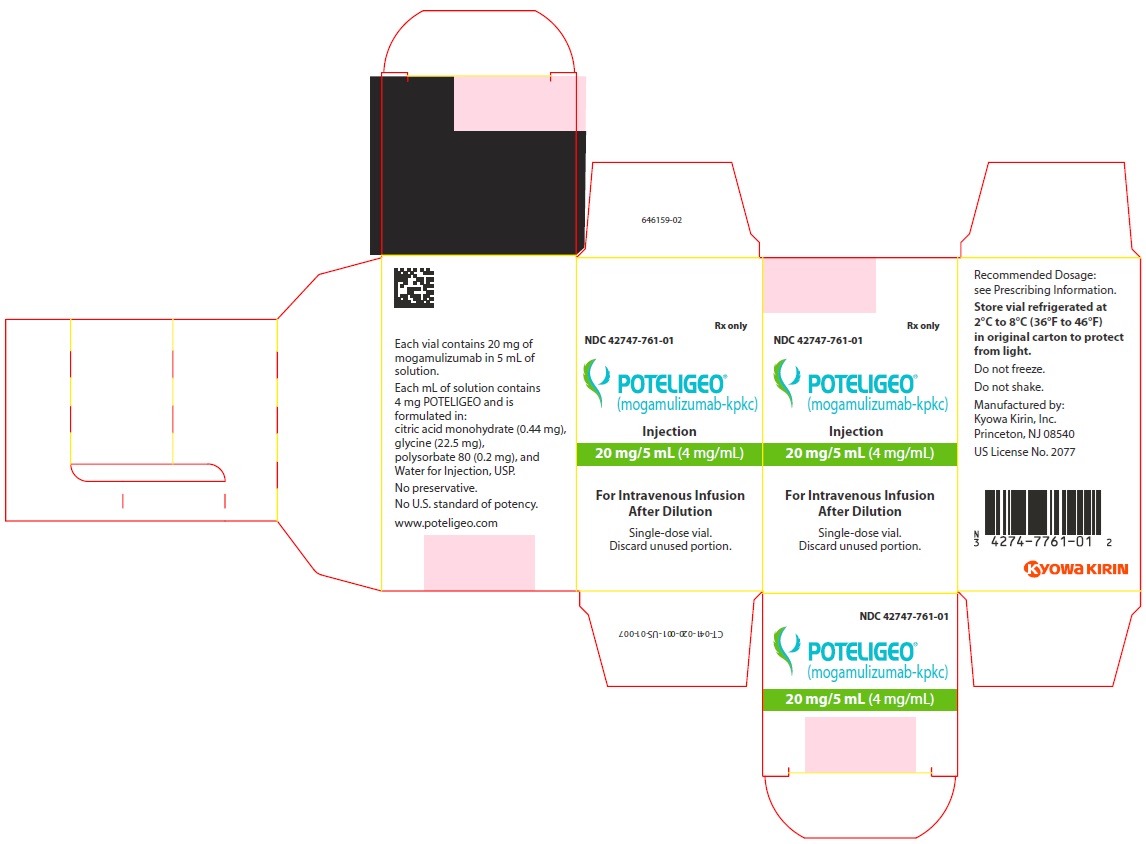
3 DOSAGE FORMS AND STRENGTHSInjection: 20 mg/5 mL (4 mg/mL) as a clear to slightly opalescent colorless solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Dermatologic Toxicity - Fatal and life-threatening skin adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have occurred in recipients of ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the labeling: Dermatologic Toxicity [see Warnings and Precautions (5.1)]. Infusion Reactions [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on POTELIGEO use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In an animal reproduction ...
-
11 DESCRIPTIONMogamulizumab-kpkc is a recombinant humanized monoclonal antibody that targets CC chemokine receptor 4 (CCR4)-expressing cells. Mogamulizumab-kpkc is an IgG1 kappa immunoglobulin that has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mogamulizumab-kpkc is a defucosylated, humanized IgG1 kappa monoclonal antibody that binds to CCR4, a G protein-coupled receptor for CC chemokines that is involved in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with POTELIGEO. No specific studies have been conducted to evaluate ...
-
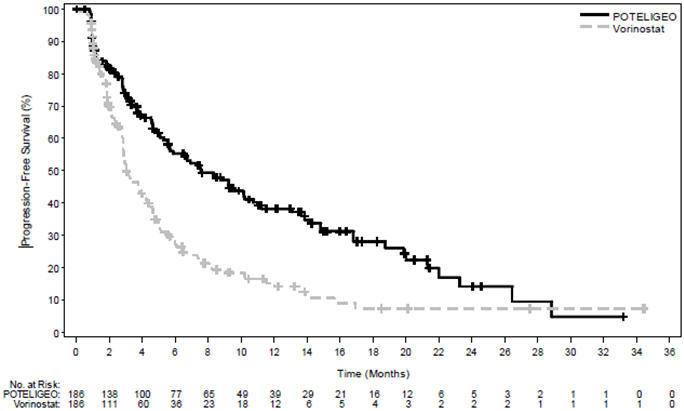
14 CLINICAL STUDIESTrial 1 - A randomized, open-label, multicenter trial (Study 0761-010; NCT01728805) evaluated the efficacy of POTELIGEO in adult patients with MF or SS after at least one prior systemic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPOTELIGEO (mogamulizumab-kpkc) injection is a sterile, preservative-free, clear to slightly opalescent colorless solution supplied in a carton containing one 20 mg/5 mL (4 mg/mL), single-dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients of the risk of the following adverse reactions that may require additional treatment and/or ...
-
SPL UNCLASSIFIED SECTIONPOTELIGEO® (mogamulizumab-kpkc) Manufactured by: Kyowa Kirin, Inc. Princeton, NJ 08540 - US License No. 2077
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Issued: 3/2023 - PATIENT INFORMATION - POTELIGEO® (poe–te–lig'–ee–oh) (mogamulizumab-kpkc) injection ...
-
PRINCIPAL DISPLAY PANEL - 4 mg/mL Vial CartonRx only - NDC 42747-761-01 - POTELIGEO® (mogamulizumab-kpkc) Injection - 20 mg/5 mL - (4 mg/mL) For Intravenous Infusion - Single-dose vial. Discard unused portion.
-
INGREDIENTS AND APPEARANCEProduct Information