Label: PIRNUO- mupirocin cream
- NDC Code(s): 68462-560-47
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PIRNUO CREAM safely and effectively. See full prescribing information for PIRNUO CREAM. PIRNUO TM (Mupirocin cream), for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE PIRNUO cream is indicated for the treatment of secondarily infected traumatic skin lesions (up to 10 cm in length or 100 cm2 in area) due to susceptible isolates of Staphylococcus aureus (S ...
-
2 DOSAGE AND ADMINISTRATION • For Topical Use Only. • Apply a small amount of PIRNUO cream, with a cotton swab or gauze pad, to the affected area 3 times daily for 10 days. • Cover the treated area with gauze dressing if ...
-
3 DOSAGE FORMS AND STRENGTHS PIRNUOTM Cream (Mupirocin Cream USP, 2%) is a white cream that contains 20 mg (2% w/w) of mupirocin per gram (equivalent to 21.5 mg (2.15% w/w) of mupirocin calcium, USP) in an oil- and ...
-
4 CONTRAINDICATIONS PIRNUO cream is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of PIRNUO cream.
-
5 WARNINGS AND PRECAUTIONS 5.1 Severe Allergic Reactions - Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of ...
-
6 ADVERSE REACTIONS The following adverse reactions are discussed in more detail in other sections of the labeling: • Severe Allergic Reactions [see Warnings and Precautions (5.1)] • Eye Irritation [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are insufficient human data to establish whether there is a drug-associated risk with mupirocin cream in pregnant women. Systemic absorption of mupirocin ...
-
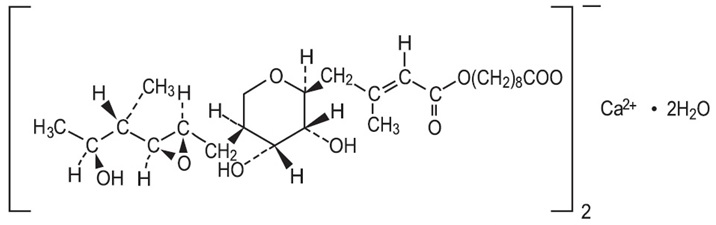
11 DESCRIPTION PIRNUOTM Cream (Mupirocin Cream USP, 2%) contains the dihydrate crystalline calcium hemi-salt of the RNA synthetase inhibitor antibacterial, mupirocin. Chemically, it is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Mupirocin is an RNA synthetase inhibitor antibacterial [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - Systemic absorption of mupirocin through ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of mupirocin calcium have not been conducted. Results of the ...
-
14 CLINICAL STUDIES The efficacy of topical mupirocin cream for the treatment of secondarily infected traumatic skin lesions (e.g., lacerations, sutured wounds, and abrasions not more than 10 cm in length or 100 cm2 ...
-
15 REFERENCES 1. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26. Clinical and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING PIRNUOTM Cream (Mupirocin Cream USP, 2%) is a white cream that contains 20 mg (2% w/w) of mupirocin per gram (equivalent to 2.15% w/w mupirocin calcium, USP) in an oil- and water-based ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Advise the patient to administer PIRNUO cream as follows: • Use PIRNUO cream only as directed by the ...
-
PATIENT INFORMATION PIRNUO (Purr-NEW – oh) (mupirocin calcium) cream - What is PIRNUO cream? PIRNUO cream is a prescription medicine used on the skin (topical use) to treat certain skin infections caused by ...
-
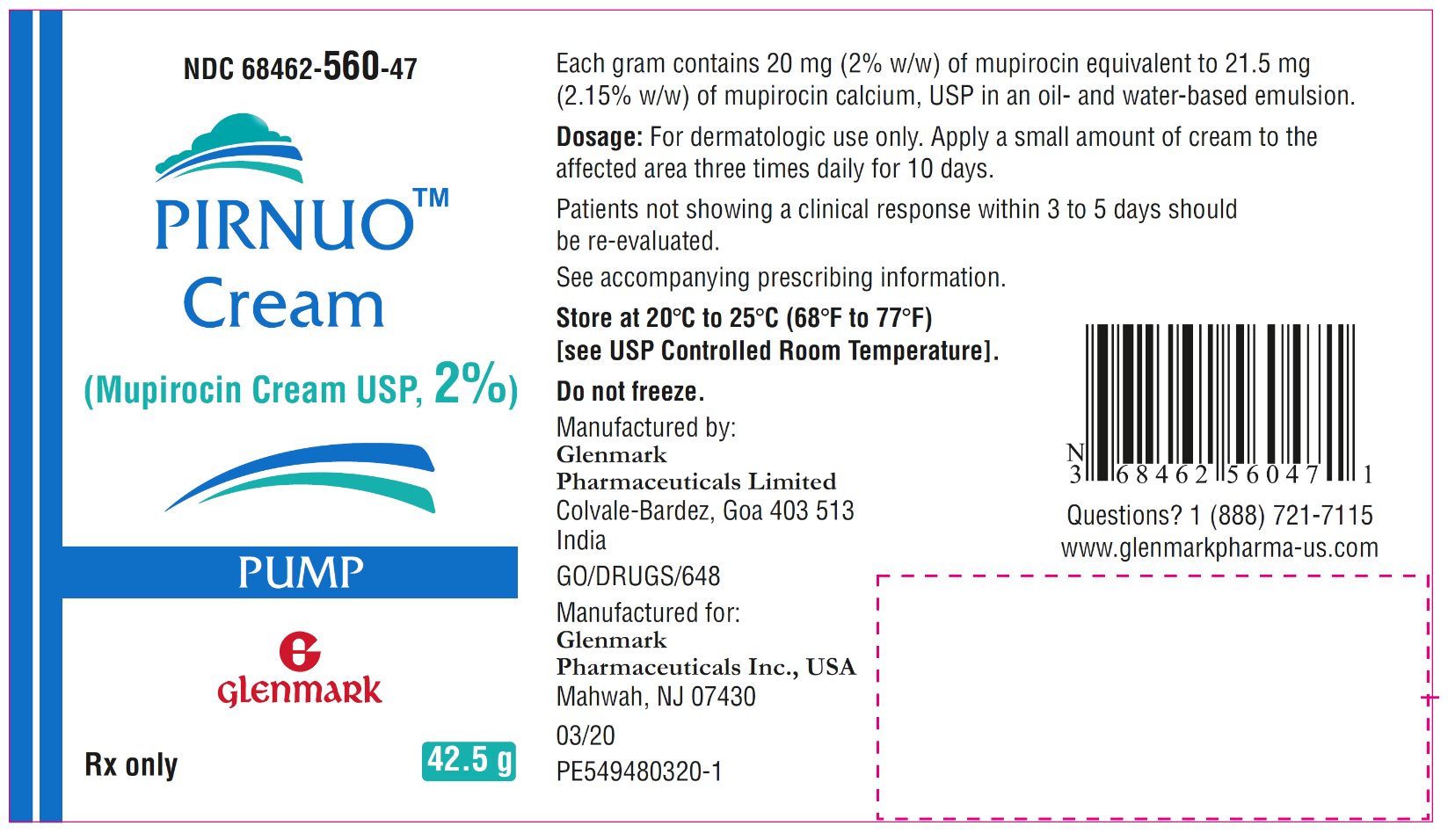
Package/Label Display Panel NDC-68462-560-47 - PIRNUO Cream - 42.5 – Carton – Pump Pack
-
Package/Label Display Panel NDC-68462-560-47 - PIRNUO Cream - 42.5 – Container Label – Pump Pack
-
INGREDIENTS AND APPEARANCEProduct Information