Label: NUPLAZID- pimavanserin tartrate capsule
NUPLAZID- pimavanserin tartrate tablet, coated
- NDC Code(s): 63090-100-30, 63090-340-30
- Packager: Acadia Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NUPLAZID safely and effectively. See full prescribing information for NUPLAZID. NUPLAZID® (pimavanserin) capsules, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. NUPLAZID is not approved for the treatment of patients with dementia who experience psychosis unless their hallucinations and delusions are related to Parkinson's disease [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGENUPLAZID® is indicated for the treatment of hallucinations and delusions associated with Parkinson's disease psychosis [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of NUPLAZID is 34 mg taken orally once daily, without titration. 2.2 Administration Information - NUPLAZID can be taken with or without food [see ...
-
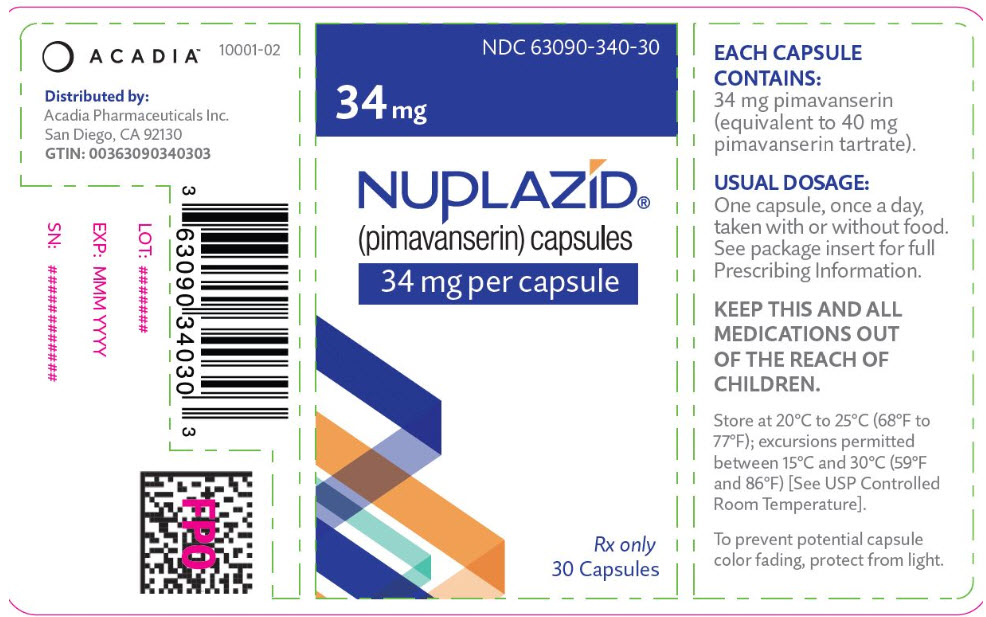
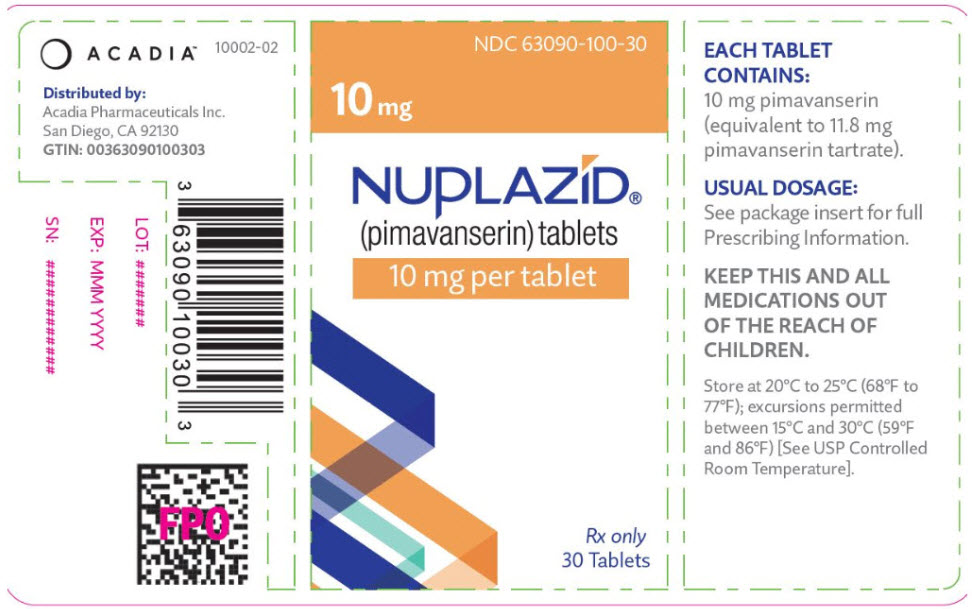
3 DOSAGE FORMS AND STRENGTHSNUPLAZID (pimavanserin) is available as: 34 mg strength capsules. The capsules are opaque white and light green with "PIMA" and "34" printed in black. 10 mg strength tablets. The orange, round ...
-
4 CONTRAINDICATIONSNUPLAZID is contraindicated in patients with a history of a hypersensitivity reaction to pimavanserin or any of its components. Rash, urticaria, and reactions consistent with angioedema (e.g. ...
-
5 WARNINGS AND PRECAUTIONS5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis - Antipsychotic drugs increase the all-cause risk of death in elderly patients with dementia-related psychosis ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning and Warnings and ...
-
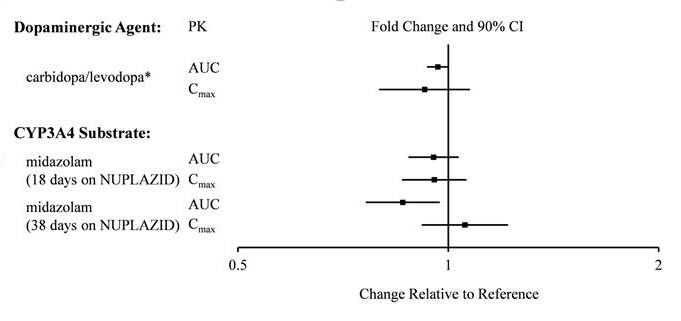
7 DRUG INTERACTIONS7.1 Drugs Having Clinically Important Interactions with NUPLAZID - Table 2 Clinically Important Drug Interactions with NUPLAZID - QT Interval Prolongation - Clinical Impact:Concomitant use ...
-
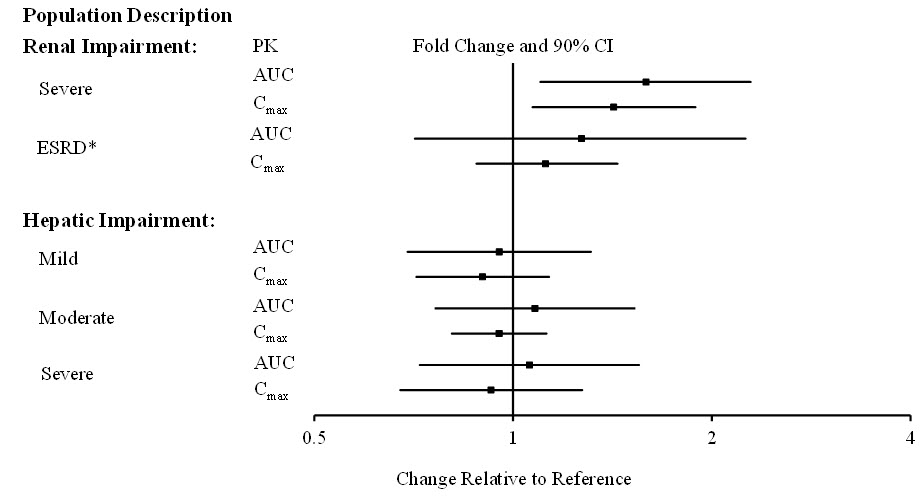
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on NUPLAZID use in pregnant women that would allow assessment of the drug-associated risk of major congenital malformations or miscarriage. In ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - NUPLAZID is not a controlled substance. 9.2 Abuse - NUPLAZID has not been systematically studied in humans for its potential for abuse, tolerance, or physical ...
-
10 OVERDOSAGE10.1 Human Experience - The pre-marketing clinical trials involving NUPLAZID in approximately 1200 subjects and patients do not provide information regarding symptoms with overdose. In healthy ...
-
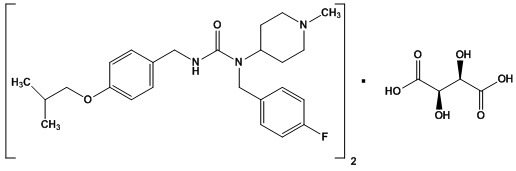
11 DESCRIPTIONNUPLAZID contains pimavanserin, an atypical antipsychotic, which is present as pimavanserin tartrate salt with the chemical name, urea ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of pimavanserin in the treatment of hallucinations and delusions associated with PDP is unclear. However, the effect of pimavanserin could be ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - There was no increase in the incidence of tumors following daily oral administration of pimavanserin to mice or ...
-
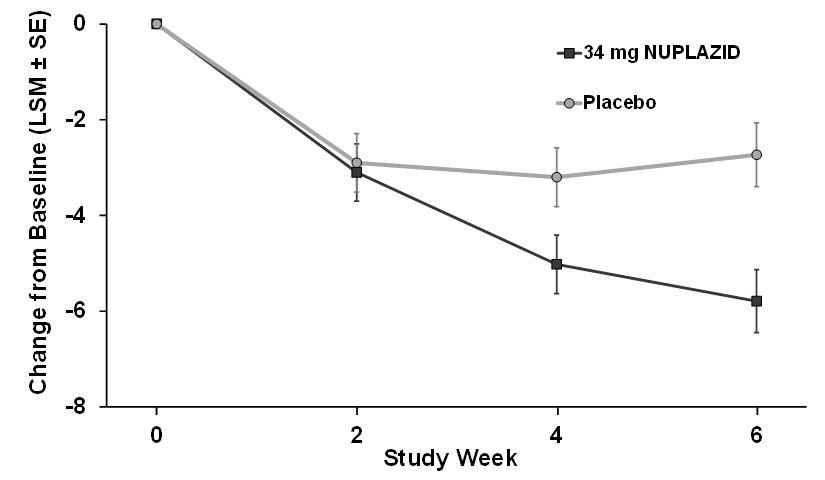
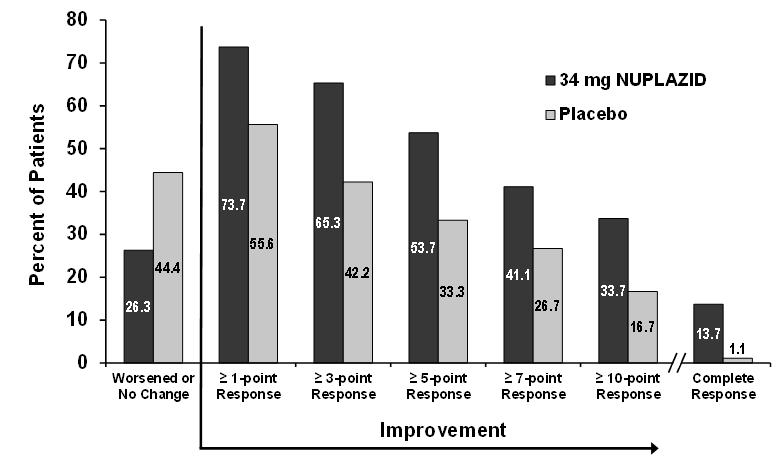
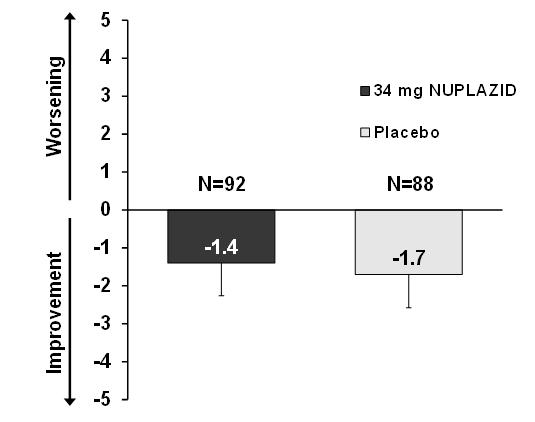
14 CLINICAL STUDIESThe efficacy of NUPLAZID 34 mg as a treatment of hallucinations and delusions associated with Parkinson's disease (PD) psychosis was demonstrated in a 6-week, randomized, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNUPLAZID (pimavanserin) is available as: 34 mg Capsule: Opaque white and light green capsule with "PIMA" and "34" printed in black. Bottle of 30: NDC 63090-340-30 - 10 mg Tablet: Orange ...
-
17 PATIENT COUNSELING INFORMATIONConcomitant Medication - Advise patients to inform their healthcare providers if there are any changes to their current prescription or over-the-counter medications, since there is a potential ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA - NUPLAZID® is a registered trademark of Acadia Pharmaceuticals Inc. ©2025 Acadia Pharmaceuticals Inc. All rights reserved.
-
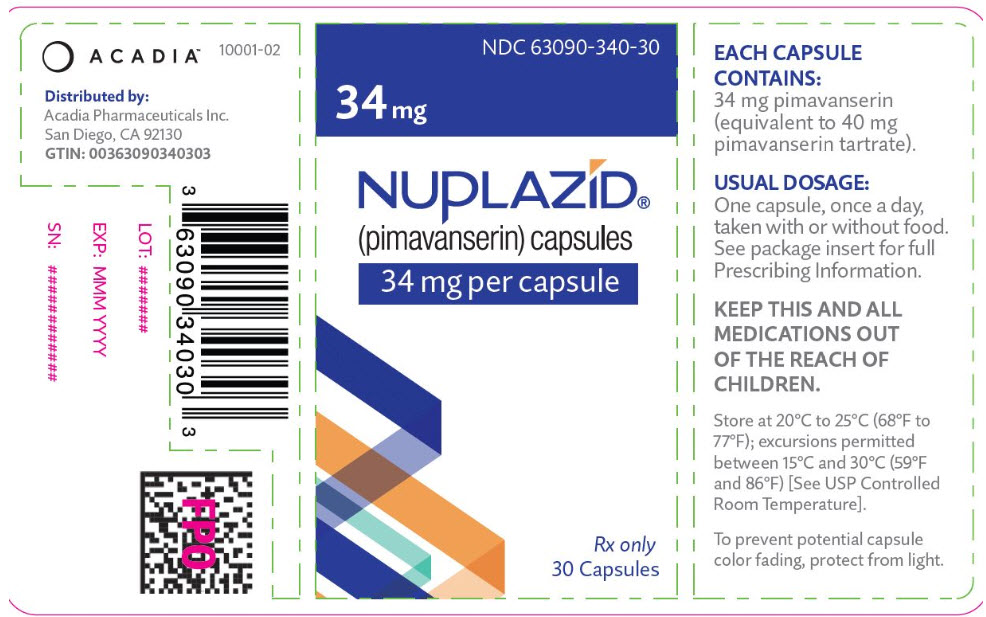
PRINCIPAL DISPLAY PANEL - 34 mg Capsule Bottle LabelNDC 63090-340-30 - 34 mg - NUPLAZID® (pimavanserin) capsules - 34 mg per capsule - Rx only - 30 Capsules
-
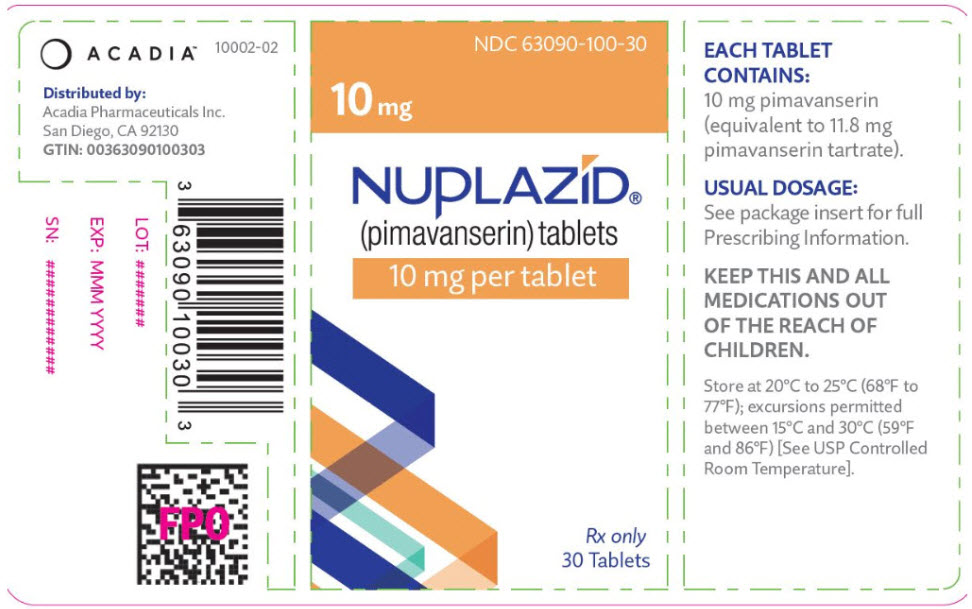
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle LabelNDC 63090-100-30 - 10 mg - NUPLAZID® (pimavanserin) tablets - 10 mg per tablet - Rx only - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information