Label: PHOTREXA VISCOUS- riboflavin 5-phosphate in 20% dextran ophthalmic solution/ drops
PHOTREXA- riboflavin 5-phosphate ophth...view full title
PHOTREXA- riboflavin 5-phosphate ophth...
- NDC Code(s): 25357-022-01, 25357-023-01, 25357-025-03
- Packager: Glaukos Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PHOTREXA® VISCOUS and PHOTREXA® safely and effectively. See full prescribing information for PHOTREXA VISCOUS and PHOTREXA ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGEPHOTREXA® VISCOUS and PHOTREXA® are indicated for use in corneal collagen cross-linking in combination with the KXL™ System for the treatment of - 1.1 Progressive keratoconus - 1.2 Corneal ...
-
2. DOSAGE AND ADMINISTRATIONUsing topical anesthesia, debride the epithelium to a diameter of approximately 9 mm using standard aseptic technique. Post epithelial debridement, instill 1 drop of PHOTREXA VISCOUS topically on ...
-
3. DOSAGE FORMS AND STRENGTHS3.1 PHOTREXA VISCOUS - PHOTREXA VISCOUS in a 3 mL glass syringe containing sterile 1.56 mg/mL riboflavin 5’-phosphate in 20% dextran ophthalmic solution for topical administration. 3.2 ...
-
4. CONTRAINDICATIONSNone.
-
5. WARNINGS AND PRECAUTIONSUlcerative keratitis can occur. Monitor for resolution of epithelial defects. [See Adverse Reactions (6)].
-
6. ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Ulcerative keratitis [Warnings and Precautions (5)] 6.1 Clinical Trials Experience - Because ...
-
8. USE IN SPECIFIC POPULATIONS8.1. Pregnancy - Risk Summary - Animal development and reproduction studies have not been conducted with the PHOTREXA® VISCOUS/PHOTREXA®/KXL® System. Since it is not known whether the corneal ...
-
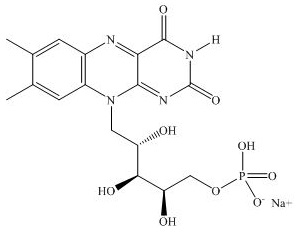
11. DESCRIPTIONPHOTREXA VISCOUS (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) 0.146% and PHOTREXA (riboflavin 5’-phosphate ophthalmic solution) 0.146% are intended for topical ophthalmic ...
-
12. CLINICAL PHARMACOLOGY12.1. Mechanism of Action - Riboflavin 5’-phosphate sodium (Vitamin B2) is the precursor of two coenzymes, flavin adenine dinucleotide and flavin mononucleotide, which catalyze ...
-
13. NONCLINICAL TOXICOLOGY13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to determine the carcinogenic potential of photoexcited riboflavin. Photoexcited riboflavin ...
-
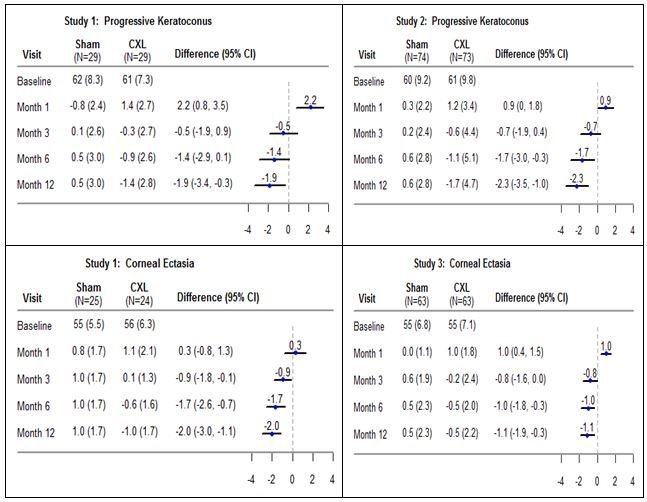
14. CLINICAL STUDIESThree prospective, randomized, parallel-group, open-label, sham-controlled trials were conducted to evaluate the safety and effectiveness of riboflavin ophthalmic solution/UVA irradiation for ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGSingle-use foil pouches of PHOTREXA® VISCOUS and PHOTREXA® are provided in a kit of two (2): one (1) PHOTREXA® VISCOUS and one (1) PHOTREXA® (NDC- 25357-025-03). Each foil pouch contains a 3 mL ...
-
17. PATIENT COUNSELING INFORMATIONPatients should be advised not to rub their eyes for the first five days after their procedure. Patients may be sensitive to light and have a foreign body sensation. Patients should be advised ...
-
PRINCIPAL DISPLAY PANEL - Photrexa Viscous Syringe Label

-
PRINCIPAL DISPLAY PANEL - Photrexa Viscous Foil Pouch Label

-
PRINCIPAL DISPLAY PANEL - Photrexa Viscous Tyvek Pouch Label

-
PRINCIPAL DISPLAY PANEL - Photrexa Syringe Label

-
PRINCIPAL DISPLAY PANEL - Photrexa Foil Pouch Label

-
PRINCIPAL DISPLAY PANEL - Photrexa Tyvek Pouch Label

-
PRINCIPAL DISPLAY PANEL - NDC 25357-025-03 - Photrexa/Photrexa Viscous Single Treatment Kit Label

-
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
PHOTREXA VISCOUS- riboflavin 5-phosphate in 20% dextran ophthalmic solution/ drops
PHOTREXA- riboflavin 5-phosphate ophth...view full title
PHOTREXA- riboflavin 5-phosphate ophth...
Number of versions: 11
RxNorm
PHOTREXA VISCOUS- riboflavin 5-phosphate in 20% dextran ophthalmic solution/ drops
PHOTREXA- riboflavin 5-phosphate ophth...view full title
PHOTREXA- riboflavin 5-phosphate ophth...
Get Label RSS Feed for this Drug
PHOTREXA VISCOUS- riboflavin 5-phosphate in 20% dextran ophthalmic solution/ drops
PHOTREXA- riboflavin 5-phosphate ophth...view full title
PHOTREXA- riboflavin 5-phosphate ophth...
NDC Codes
PHOTREXA VISCOUS- riboflavin 5-phosphate in 20% dextran ophthalmic solution/ drops
PHOTREXA- riboflavin 5-phosphate ophth...view full title
PHOTREXA- riboflavin 5-phosphate ophth...